GENOMIC
Mapping
14qE2.3. View the map and BAC clones (data from UCSC genome browser).
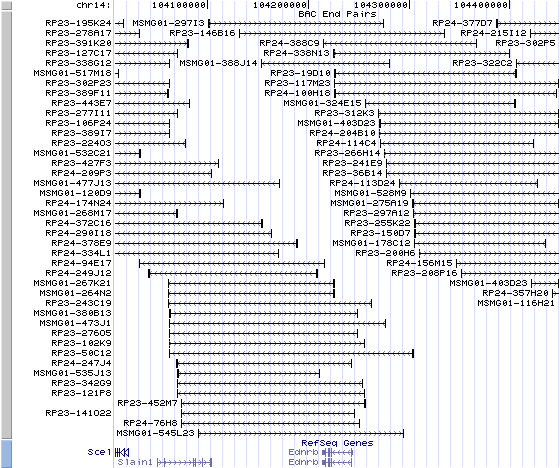
Structure
(assembly 02/2006)
Ednrb (NM_007904): 7 exons, 29,071 bp, chr14:102700305-102729375.
The figure below shows the structure of the Ednrb gene (data from UCSC genome browser).

Regulatory Element
Search the 5'UTR and 1kb upstream regions (seq1=mouse Ednrb, seq2=human EDNRB) by CONREAL with 80% Position Weight Matrices (PWMs) threshold (view results here).
TRANSCRIPT
RefSeq/ORF
Ednrb (NM_007904), 3,990 bp, view ORF and the alignment to genomic.
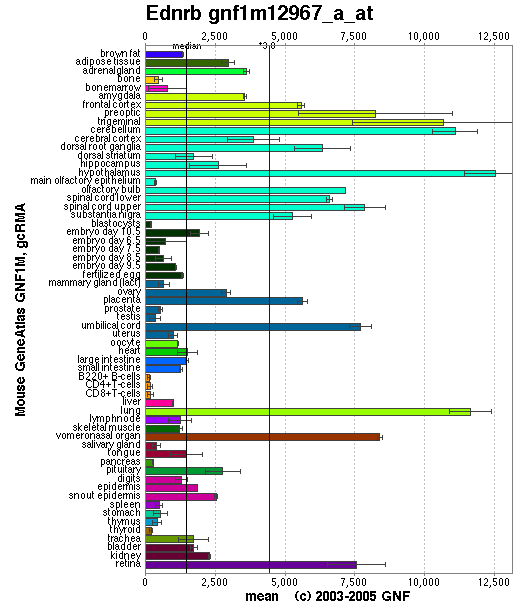
Expression Pattern
Affymetrix microarray expression pattern in SymAtlas from GNF is shown below.
Browse more information in Entrez Gene, UCSC Gene Sorter, MGI.
PROTEIN
Sequence
endothelin receptor type B (NP_031930): 442 aa, UniProtKB/Swiss-Prot entry P48302.
Ortholog
Species
Human Chimpanzee Dog Rat Fowl GeneView
EDNRB
EDNRB
EDNRB
Ednrb
EDNRB
Protein
NP_000106 (442 aa)
XP_509693 (532 aa)
NP_001010943 (442 aa)
NP_059029 (442 aa)
XP_41700 (560 aa)
Identities
393/443 (88%) 393/443 (88%) 391/443 (88%) 431/442 (97%) 339/435 (77%)
View multiple sequence alignment (PDF file) by ClustalW and GeneDoc. View evolutionary tree by TreeView.
Domain
(1) Domains predicted by SMART:
a) signal peptide: 1-26
b) Pfam:7tm_1: 118-386
(2) Transmembrane domains predicted by SOSUI:
This amino acid sequence is of a MEMBRANE PROTEIN
which have 8 transmembrane helices.
No. N terminal transmembrane region C terminal type length 1 6 SRCGRALVALLLACGFLGVWGE 27 PRIMARY 22 2 100 FKYINTIVSCLVFVLGIIGNSTL 122 PRIMARY 23 3 138 ILIASLALGDLLHIIIDIPINTY 160 SECONDARY 23 4 173 MCKLVPFIQKASVGITVLSLCAL 195 SECONDARY 23 5 220 VEIVLIWVVSVVLAVPEAIGFDM 242 PRIMARY 23 6 277 LFSFYFCLPLAITAVFYTLMTCE 299 SECONDARY 23 7 325 VFCLVLVFALCWLPLHLSRILKL 347 PRIMARY 23 8 358 CELLSFLLVLDYIGINMASLNSC 380 SECONDARY 23
(3) Graphic view of InterPro domain structure.
Motif/Site
(1) Predicted results by ScanProsite:
a)N-glycosylation site:
Site : 60 to 63 NSSL.
Site : 119 to 122 NSTL.
b) cAMP- and cGMP-dependent protein kinase phosphorylation site:
Site : 302 to 305 RKKS.
c) Protein kinase C phosphorylation site:
Site : 99 to 101 TFK.
Site : 159 to 161 TYK.
Site : 271 to 273 TAK.
Site : 390 to 392 SKR.
Site : 436 to 438 SNK.
d) Casein kinase II phosphorylation site:
Site : 218 to 221 TAVE.
Site : 271 to 274 TAKD.
Site : 348 to 351 TLYD.
Site : 407 to 410 TFEE.
Site : 413 to 416 SLEE.
e)Tyrosine kinase phosphorylation site:
Site : 424 to 430 KANDHGY.
f) N-myristoylation site:
Site : 58 to 63 GSNSSL.
Site : 75 to 80 GGRGAG.
Site : 115 to 120 GIIGNS.
Site : 170 to 175 GAEMCK.
Site : 306 to 311 GMQIAL.
Site : 371 to 376 GINMAS.
g) G-protein coupled receptors family 1 signature:
Site : 187 to 203 ITVLSLCALSIDRYRAV.
(2) Predicted results of subprograms by PSORT II:
a) Seems to have a cleavable signal peptide (1 to 26)
b) Tentative number of TMS(s) for the threshold 0.5: 7
INTEGRAL Likelihood = -5.04 Transmembrane 102 - 118
INTEGRAL Likelihood = -3.61 Transmembrane 137 - 153
INTEGRAL Likelihood = -0.37 Transmembrane 179 - 195
INTEGRAL Likelihood = -8.92 Transmembrane 218 - 234
INTEGRAL Likelihood = -2.76 Transmembrane 276 - 292
INTEGRAL Likelihood = -8.07 Transmembrane 325 - 341
INTEGRAL Likelihood = -0.80 Transmembrane 360 - 376
PERIPHERAL Likelihood = 8.01 (at 377)
ALOM score: -8.92 (number of TMSs: 7)
c) KDEL ER retention motif in C-terminus: none
d) ER membrane retention signals: KYSS
e) VAC possible vacuolar targeting motif: none
f) Actinin-type actin-binding motif: type 1: none; type 2: none
g) Prenylation motif: none
h) memYQRL transport motif from cell surface to Golgi: none
i) Tyrosines in the tail: none
j) Dileucine motif in the tail: none
3D Model
ModBase predicted 3D structure from UCSC Gene Sorter:
P48302 (endothelin receptor type B):
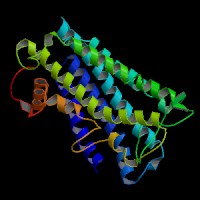
From left to right: Front, Top, and Side views of predicted protein.
2D-PAGE
This protein does not exist in the current release of SWISS-2DPAGE.
Computed theoretical MW=49,560.7Da, pI=9.43.
| Species | Human | Chimpanzee | Dog | Rat | Fowl |
| GeneView | EDNRB | EDNRB | EDNRB | Ednrb | EDNRB |
| Protein | NP_000106 (442 aa) | XP_509693 (532 aa) | NP_001010943 (442 aa) | NP_059029 (442 aa) | XP_41700 (560 aa) |
| Identities | 393/443 (88%) | 393/443 (88%) | 391/443 (88%) | 431/442 (97%) | 339/435 (77%) |
View multiple sequence alignment (PDF file) by ClustalW and GeneDoc. View evolutionary tree by TreeView.
Domain
(1) Domains predicted by SMART:
a) signal peptide: 1-26
b) Pfam:7tm_1: 118-386
(2) Transmembrane domains predicted by SOSUI:
This amino acid sequence is of a MEMBRANE PROTEIN
which have 8 transmembrane helices.
| No. | N terminal | transmembrane region | C terminal | type | length |
| 1 | 6 | SRCGRALVALLLACGFLGVWGE | 27 | PRIMARY | 22 |
| 2 | 100 | FKYINTIVSCLVFVLGIIGNSTL | 122 | PRIMARY | 23 |
| 3 | 138 | ILIASLALGDLLHIIIDIPINTY | 160 | SECONDARY | 23 |
| 4 | 173 | MCKLVPFIQKASVGITVLSLCAL | 195 | SECONDARY | 23 |
| 5 | 220 | VEIVLIWVVSVVLAVPEAIGFDM | 242 | PRIMARY | 23 |
| 6 | 277 | LFSFYFCLPLAITAVFYTLMTCE | 299 | SECONDARY | 23 |
| 7 | 325 | VFCLVLVFALCWLPLHLSRILKL | 347 | PRIMARY | 23 |
| 8 | 358 | CELLSFLLVLDYIGINMASLNSC | 380 | SECONDARY | 23 |
(3) Graphic view of InterPro domain structure.
Motif/Site
(1) Predicted results by ScanProsite:
a)N-glycosylation site:
Site : 60 to 63 NSSL.
Site : 119 to 122 NSTL.
b) cAMP- and cGMP-dependent protein kinase phosphorylation site:
Site : 302 to 305 RKKS.
c) Protein kinase C phosphorylation site:
Site : 99 to 101 TFK.
Site : 159 to 161 TYK.
Site : 271 to 273 TAK.
Site : 390 to 392 SKR.
Site : 436 to 438 SNK.
d) Casein kinase II phosphorylation site:
Site : 218 to 221 TAVE.
Site : 271 to 274 TAKD.
Site : 348 to 351 TLYD.
Site : 407 to 410 TFEE.
Site : 413 to 416 SLEE.
e)Tyrosine kinase phosphorylation site:
Site : 424 to 430 KANDHGY.
f) N-myristoylation site:
Site : 58 to 63 GSNSSL.
Site : 75 to 80 GGRGAG.
Site : 115 to 120 GIIGNS.
Site : 170 to 175 GAEMCK.
Site : 306 to 311 GMQIAL.
Site : 371 to 376 GINMAS.
g) G-protein coupled receptors family 1 signature:
Site : 187 to 203 ITVLSLCALSIDRYRAV.
(2) Predicted results of subprograms by PSORT II:
a) Seems to have a cleavable signal peptide (1 to 26)
b) Tentative number of TMS(s) for the threshold 0.5: 7
INTEGRAL Likelihood = -5.04 Transmembrane 102 - 118
INTEGRAL Likelihood = -3.61 Transmembrane 137 - 153
INTEGRAL Likelihood = -0.37 Transmembrane 179 - 195
INTEGRAL Likelihood = -8.92 Transmembrane 218 - 234
INTEGRAL Likelihood = -2.76 Transmembrane 276 - 292
INTEGRAL Likelihood = -8.07 Transmembrane 325 - 341
INTEGRAL Likelihood = -0.80 Transmembrane 360 - 376
PERIPHERAL Likelihood = 8.01 (at 377)
ALOM score: -8.92 (number of TMSs: 7)
c) KDEL ER retention motif in C-terminus: none
d) ER membrane retention signals: KYSS
e) VAC possible vacuolar targeting motif: none
f) Actinin-type actin-binding motif: type 1: none; type 2: none
g) Prenylation motif: none
h) memYQRL transport motif from cell surface to Golgi: none
i) Tyrosines in the tail: none
j) Dileucine motif in the tail: none
3D Model
ModBase predicted 3D structure from UCSC Gene Sorter:
P48302 (endothelin receptor type B):



2D-PAGE
This protein does not exist in the current release of SWISS-2DPAGE.
Computed theoretical MW=49,560.7Da, pI=9.43.
FUNCTION
Ontology
(1) Biological process: neural crest cell migration, signal transduction, smooth muscle contraction, sensory perception of sound.
(2) Regulation of blood pressure.
(3) G-protein coupled receptor activity, rhodopsin-like.
(4) Inositol phosphate-mediated signaling.
(5) Melanocyte differentiation.
Location
Plasma membrane.
Interaction
The protein encoded by this gene is a G protein-coupled receptor which activates a phosphatidylinositol-calcium second messenger system. Its ligand, endothelin, consists of a family of three potent vasoactive peptides: ET1, ET2, and ET3. While both isoforms bind ET1, they exhibit different responses upon binding, suggesting that they may be functionally distinct. The interaction of this endothelin with EDNRB is essential for development of neural crest-derived cell lineages, such as melanocytes and enteric neurons.
View interactions in HPRD
View co-occured partners in literature searched by PPI Finder.
Pathway
Neuroactive ligand-receptor interaction, Melanogenesis in KEGG.
MUTATION
Allele or SNP
38 phenotypic alleles of Ednrb are described in MGI:102720.
SNPs deposited in dbSNP Build 128.
Distribution
Among the 38 reported alleles, 3 are spontaneous, 2 are targeted knock-outs, 10 are ENU-induced.
(Numbering of cDNA sequence is based on the start codon of RefSeq NM_007904. view ORF here.)
Effect
Null mutations in the mouse genes Ednrb affect the pathway in the normal development of melanocytes and other neural crest-derived lineages.
PHENOTYPE
Homozygotes for null mutations have pigmentation limited to small patches on the head and rump, exhibit abnormal neural epithelium of the inner ear, and die from megacolon resulting from impaired neuronal migration and aganglia.
Phenotype analysis of Sox10;Ednrb and Sox10;Edn3 double mutants showed that a coordinate and balanced interaction between these molecules is required for normal enteric nervous system (ENS) and melanocyte development. Double mutants present with a severe increase in white spotting, absence of melanocytes within the inner ear, and in the stria vascularis in particular, and more severe ENS defects (Stanchina, et al.).
REFERENCE
- Stanchina L, Baral V, Robert F, Pingault V, Lemort N, Pachnis V, Goossens M, Bondurand N. Interactions between Sox10, Edn3 and Ednrb during enteric nervous system and melanocyte development. Dev Biol 2006; 295:232-49. PMID: 16650841