GENOMIC
Mapping
2q37.3. View the map and BAC clones (data from UCSC genome browser).
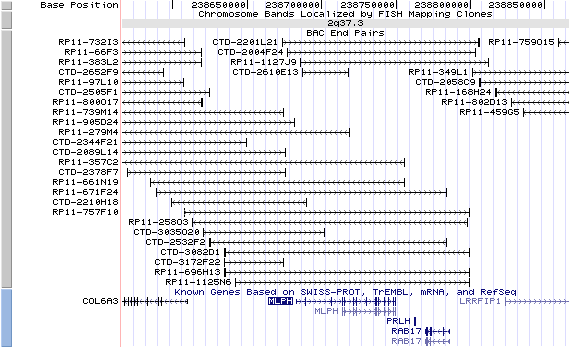
Structure
(assembly 07/03)
MLPH/NM_024101: 16 exons, 66,707bp, Chr2: 238,682,680-238,749,386.
The figure below shows the structure of the MLPH gene (data from UCSC genome browser).

Regulatory Element
Search the 5'UTR and 1kb upstream regions (human and mouse) by CONREAL with 80% Position Weight Matrices (PWMs) threshold (view results here).
TRANSCRIPT
RefSeq/ORF
MLPH (NM_024101), 2,403bp, view ORF and the alignment to genomic.
Expression Pattern
Tissue specificity: expressed in melanocytes, highest in prostate tissue. Not detectable in CTL cells (Hume, et al).
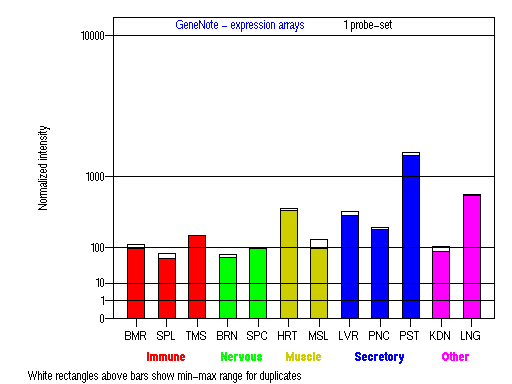
BMR: Bone marrow; SPL: Spleen; TMS: Thymus; BRN: Brain; SPC: Spinal cord; HRT: Heart; MSL: Skeletal muscle;
LVR; Liver; PNC: Pancreas; PST: Prostate; KDN: Kidney; LNG: Lung. (data from GeneCards )
PROTEIN
Sequence
Melanophilin (NP_077006): 600aa, ExPaSy NiceProt view of Swiss-Prot:Q9BV36.
Synonyms: Leaden homolog; Exophilin 3; Synaptotagmin-like protein 2a; Slp homologue lacking C2 domains-a; SLAC2-A
Ortholog
| Species | Mouse | Rat | Chicken | Fugufish |
| GeneView | ln/Mlph | LOC316620 | 03904 | 156198 |
| Protein | NP_443748 (590aa) | XP_237388 (682aa) | 06194 (593aa) | 175372 (401aa) |
| Identities | 63%/386aa | 63%/347aa | 46%/289aa | 34%/75aa |
View multiple sequence alignment (PDF file) by ClustalW and GeneDoc.
Domain
(1) Domains predicted by SMART:
a) coiled coil 27 - 56
b) coiled coil 374 - 409
c) coiled coil 457 - 496
(2) Transmembrane domains predicted by SOSUI: None.
(3) Graphic view of InterPro domain structure.
(4) CDD domain: Pfam02318: Rabphilin-3A effector domain.
Motif/Site
(1) Predicted results by ScanProsite:
a) Rab-binding domain profile :
4 - 124: score=13.530
b) Amidation site : [occurs frequently]
1 - 4: mGKK,
523 - 526: lGKR.
c) cAMP- and cGMP-dependent protein kinase phosphorylation site : [occurs frequently]
44 - 47: KKeS,
507 - 510: RRkS,
549 - 552: RKfS.
d) N-glycosylation site : [occurs frequently]
60 - 63: NETH,
395 - 398: NVSD,
455 - 458: NRTT.
e) N-myristoylation site : [occurs frequently]
85 - 90: GLftCK,
243 - 248: GLeeAD,
250 - 255: GAsgCH,
288 - 293: GTaaAL,
369 - 374: GLgaGV
f) Bipartite nuclear targeting sequence : [occurs frequently]
29 - 45:
RRkeeerlealkgkikk,
30 - 46:
RKeeerlealkgkikke.
(2) Predicted results of subprograms by PSORT II:
a) N-terminal signal peptide: none
b) KDEL ER retention motif in the C-terminus: none
c) ER Membrane Retention Signals: none
d) VAC possible vacuolar targeting motif: none
e) Actinin-type actin-binding motif: type 1: none; type 2: none
f) Prenylation motif: none
g) memYQRL transport motif from cell surface to Golgi: none
h) Tyrosines in the tail: none
i) Dileucine motif in the tail: none
3D Model
(1) ModBase entry found, results here.
(2)ModBase predicted comparative 3D structure of Q9BV36 from UCSC Genome Sorter.
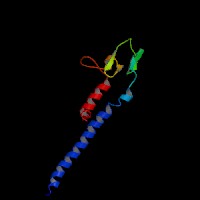
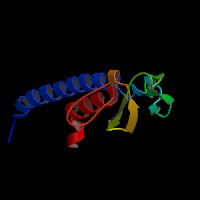
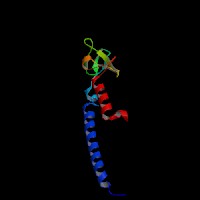
From left to right: Front, Top, and Side views of predicted protein.
2D-PAGE
This protein does not exist in the current release of SWISS-2DPAGE.
Computed theoretical MW=65,949Da, pI=5.73 (NP_077006).
FUNCTION
Ontology
a) Biological process: melanosome transport
b) Rab27A effector protein, actin binding, myosin binding
Location
Cytoplasmic.
Interaction
Melanophilin is one of the RAB27A effectors or a member of the exophilins or Slp/Slac2 family. It interacts with RAB27A via a conserved Rab27-binding domain, the Slp homology domain (SHD) (Strom, et al). The N-terminal SHD consists of two conserved alpha-helical regions (SHD1 and SHD2) that are often separated by two zinc finger motifs. SHD1 of melanophilin alone is both necessary and sufficient for high affinity specific recognition of the GTP-bound form of RAB27A. By contrast, the zinc finger motifs and SHD2 seem to be important for stabilization of the structure of the SHD or higher affinity RAB27A binding (Fukuda, et al (2002a); Nagashima, et al).
Slac2-a/melanophilin and Slac2-c/MyRIP are linker proteins between Rab27a and myosin Va. Slac2-a directly interacts with Rab27a and myosin Va via its N-terminal region (amino acids 1 to 146) and the middle region (amino acids 241 to 405), respectively (Fukuda, et al (2002b)). Melanophilin is required with Rab27a to recruit myosin Va to melanosomes in melanocytes (Hume, et al). Rab27a binds to the melanosome first and then recruits melanophilin, which in turn recruits myosin-Va. Melanophilin creates this link by binding to Rab27a in a GTP-dependent fashion through its amino terminus, and to myosin-Va through its carboxy terminus (Wu, et al). GTP-hydrolysis leads to the inactivation of Rab27a and presumably to the dissociation of melanophilin and myosin Va. cAMP stimulates the expression of Rab27a and rapidly increases the interaction of the melanophilin/Slac2-a with actin, allowing the rapid accumulation of melanosomes in the actin-rich region of the dendrite extremities after the action of melanocyte-differentiating agent such as alpha-melanocyte-stimulating hormone (Passeron, et al). Melanophilin directly activates the actin-activated ATPase activity of myosin Va and thus its motor activity (Li, et al).
The most C-terminal conserved region of Slac2-a (amino acids 400-590) and Slac2-c (amino acids 670-856), which is not essential for myosin Va binding, directly binds actin. Expression of these regions in PC12 cells and melanoma cells colocalized with actin filaments at the cell periphery, suggesting a novel role of Slac2-a/c in capture of Rab27-containing organelles in the actin-enriched cell periphery (Fukuda, et al (2002c)).
No MLPH drosophila homolog shown in CuraGen interaction database.
Pathway
Involved in melanosome transport and distribution (view diagram of the Slac2-a tripartite protein complex and the dynamics of melanosomes here).
MUTATION
Allele or SNP
No mutations deposited in HGMD.
SNPs deposited in dbSNP.
No selected allelic examples described in OMIM.
Distribution
| Location | Genomic | cDNA | Protein | Type | Strain | Reference |
| Exon 2 | 103C>T | 103C>T | R35W | missense | ? | Menasche, et al |
Effect
The R35W missense mutation falls within the SHD1 of MLPH, which is directly binds to RAB27A, completely blocks the interaction with RAB27A (Menasche, et al). The clinical features of the above patient are described in Sanal, et al .
PHENOTYPE
Defects in MLPH (OMIM:606526) are a cause of Griscelli syndrome type 3 (GS3). Griscelli syndrome (OMIM:214450) is a rare autosomal recessive disorder that results in pigmentary dilution of the skin and hair (a silver-gray sheen of the hair), the presence of large clumps of pigment in hair shafts, and an accumulation of melanosomes in melanocytes. Griscelli syndrome type 3 does not present immunologic (GS2) or neurologic (GS1 or GS2) abnormalities. Mutations in MLPH may cause a phenotype restricted to hypopigmentation (Menasche, et al).
REFERENCE
- Fukuda M. Synaptotagmin-like protein (Slp) homology domain 1 of Slac2-a/melanophilin is a critical determinant of GTP-dependent specific binding to Rab27A. J Biol Chem 2002a; 277: 40118-24. PMID: 12189142
- Fukuda M, Kuroda TS, Mikoshiba K. Slac2-a/melanophilin, the missing link between Rab27 and myosin Va: implications of a tripartite protein complex for melanosome transport. J Biol Chem 2002b; 277: 12432-6. PMID: 11856727
- Fukuda M, Kuroda TS. Slac2-c (synaptotagmin-like protein homologue lacking C2 domains-c), a novel linker protein that interacts with Rab27, myosin Va/VIIa, and actin. J Biol Chem 2002c; 277: 43096-103. PMID: 12221080
- Hume AN, Collinson LM, Hopkins CR, Strom M, Barral DC, Bossi G, Griffiths GM, Seabra MC. The leaden gene product is required with Rab27a to recruit myosin Va to melanosomes in melanocytes. Traffic 2002; 3: 193-202. PMID: 11886590
- Li XD, Ikebe R, Ikebe M. Activation of Myosin Va function by melanophilin, a specific docking partner of Myosin Va. J Biol Chem 2005; 280:17815-22. PMID: 15760894
- Menasche G, Ho CH, Sanal O, Feldmann J, Tezcan I, Ersoy F, Houdusse A, Fischer A, de Saint Basile G. Griscelli syndrome restricted to hypopigmentation results from a melanophilin defect (GS3) or a MYO5A F-exon deletion (GS1). J Clin Invest 2003; 112: 450-6. PMID: 12897212
- Nagashima K, Torii S, Yi Z, Igarashi M, Okamoto K, Takeuchi T, Izumi T. Melanophilin directly links Rab27a and myosin Va through its distinct coiled-coil regions. FEBS Lett 2002; 517: 233-8. PMID: 12062444
- Passeron T, Bahadoran P, Bertolotto C, Chiaverini C, Busca R, Valony G, Bille K, Ortonne JP, Ballotti R. Cyclic AMP promotes a peripheral distribution of melanosomes and stimulates melanophilin/Slac2-a and actin association. FASEB J 2004; 18: 989-91. PMID: 15059972
- Sanal O, Ersoy F, Tezcan I, Metin A, Yel L, Menasche G, Gurgey A, Berkel I, de Saint Basile G. Griscelli disease: genotype-phenotype correlation in an array of clinical heterogeneity. J Clin Immunol 2002; 22: 237-43. PMID: 12148598
- Strom M, Hume AN, Tarafder AK, Barkagianni E, Seabra MC. A family of Rab27-binding proteins. Melanophilin links Rab27a and myosin Va function in melanosome transport.J Biol Chem 2002; 277: 25423-30. PMID: 11980908
- Wu XS, Rao K, Zhang H, Wang F, Sellers JR, Matesic LE, Copeland NG, Jenkins NA, Hammer JA 3rd. Identification of an organelle receptor for myosin-Va. Nat Cell Biol 2002; 4: 271-8. PMID: 11887186
EDIT HISTORY:
Created by Wei Li & Jonathan Bourne: 07/22/2004
Updated by Wei Li: 04/06/2006