GENOMIC
Mapping
15q21.3 View the map and BAC clones (data from UCSC genome browser).
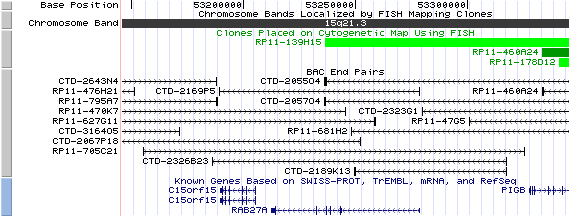
Structure
(assembly 07/03)
Isoform a (NM_004580): 6 exons, 66,784bp, Chr15: 53,211,858-53,278,641.
Isoform b (NM_183234): 7 exons, 66,683bp, Chr15: 53,211,858-53,278,540.
Isoform c (NM_183235): 7 exons, 86,200bp, Chr15: 53,211,858-53,298,057.
Isoform d (NM_183236): 7 exons, 67,306bp, Chr15: 53,211,858-53,279,163.
Note that these four alternative splicing transcript variants encode the same protein.
The figure below shows the structure of the known isoforms (data from UCSC genome browser).

Regulatory Element
Search the 5'UTR and 1kb upstream regions (human and mouse) by CONREAL with 80% Position Weight Matrices (PWMs) threshold (view results here).
TRANSCRIPT
RefSeq/ORF
a) Transcript variant 1 (NM_004580),
2,824bp, view ORF and the alignment to genomic.
b) Transcript variant 2 (NM_183234),
2,812bp, view ORF and the alignment to genomic.
c) Transcript variant 3 (NM_183235),
2,809bp, view ORF and the alignment to genomic.
d) Transcript variant 4 (NM_183236),
2,772bp, view ORF and the alignment to genomic.
Expression Pattern
Tissue specificity: Found in all the examined tissues. Low expression was found in brain, thymus, kidney, muscle and placenta. Detected in melanocytes, and in most tumor cell lines examined.
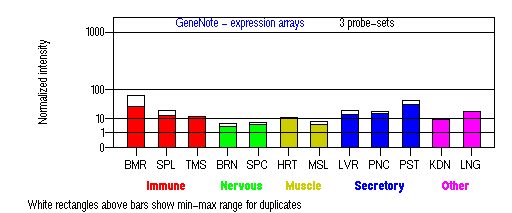
BMR: Bone marrow; SPL: Spleen; TMS: Thymus; BRN: Brain; SPC: Spinal cord; HRT: Heart; MSL: Skeletal muscle;
LVR; Liver; PNC: Pancreas; PST: Prostate; KDN: Kidney; LNG: Lung. (data from GeneCards )
PROTEIN
Sequence
Isoform a (RAB-27A/NP_899058): 221aa, ExPaSy NiceProt view of Swiss-Prot:P51159.
Note: NP_004571, NP_899057, and NP_899059 are identical to NP_899058.
Synonyms: Ras-related protein Rab-27A; GTP-binding protein Ram.
Ortholog
Species
Mouse Rat Zebrafish Worm Fruitfly GeneView
Rab27a Ram 10660 rab-27 CG14791 Protein
NP_076124 (221aa) NP_059013 (221aa) 22345 (156aa) NP_493376 (215aa) NP_569921 (230aa) Identities
95%/212aa 96%/213aa 76%/120aa 66%/127aa 63%/116aa
View multiple sequence alignment (PDF file) by ClustalW and GeneDoc.
Domain
(1) Domains predicted by SMART:
a) RAB (SM00175): 10 - 184
(2) Graphic view of the InterPro domain structure.
(3) Transmembrane domains predicted by SOSUI: None.
(4) CDD domains:
a) KOG0081: GTPase Rab27, small G protein superfamily [Intracellular trafficking, secretion, and vesicular transport].
b) KOG0070: GTP-binding ADP-ribosylation factor Arf1 [Intracellular trafficking, secretion, and vesicular transport].
Motif/Site
(1) Predicted results by ScanProsite:
a) Casein kinase II phosphorylation site : [occurs frequently]
2 - 5: SdgD,
56 - 59: SgpD,
135 - 138: SdlE.
b) ATP/GTP-binding site motif A (P-loop) : [occurs frequently]
16 - 23: GdsgvGKT.
c) N-myristoylation site : [occurs frequently]
19 - 24: GVgkTS,
167 - 172: GTniSQ,
197 - 202: GVvrSN.
d) Protein kinase C phosphorylation site : [occurs frequently]
62 - 64: TgR.
e) N-glycosylation site : [occurs frequently]
133 - 136: NKSD,
166 - 169: NGTN,
169 - 172: NISQ.
(2) Predicted results of subprograms by PSORT II:
a) N-terminal signal peptide: none
b) KDEL ER retention motif in the C-terminus: none
c) ER Membrane Retention Signals: none
d) VAC possible vacuolar targeting motif: none
e) Actinin-type actin-binding motif: type 1: none; type 2: none
f) Prenylation motif: Found CXC motif in the C-terminus: CGC
g) memYQRL transport motif from cell surface to Golgi: none
h) Tyrosines in the tail: none
i) Dileucine motif in the tail: none
3D Model
(1) ModBase entry found, results here.
(2)ModBase Predicted Comparative 3D Structure of P51159 from UCSC Genome Sorter.
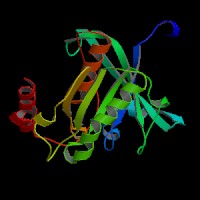
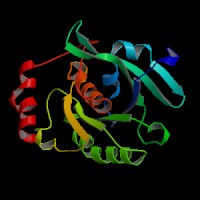
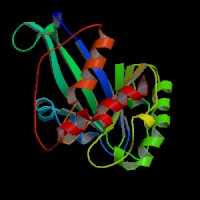
From left to right: Front, Top, and Side views of predicted protein.
2D-PAGE
This protein does not exist in the current release of SWISS-2DPAGE.
Computed theoretical MW=24,868Da, pI=5.09 (NP_899058, representative isoform).
| Species | Mouse | Rat | Zebrafish | Worm | Fruitfly |
| GeneView | Rab27a | Ram | 10660 | rab-27 | CG14791 |
| Protein | NP_076124 (221aa) | NP_059013 (221aa) | 22345 (156aa) | NP_493376 (215aa) | NP_569921 (230aa) |
| Identities | 95%/212aa | 96%/213aa | 76%/120aa | 66%/127aa | 63%/116aa |
View multiple sequence alignment (PDF file) by ClustalW and GeneDoc.
Domain
(1) Domains predicted by SMART:
a) RAB (SM00175): 10 - 184
(2) Graphic view of the InterPro domain structure.
(3) Transmembrane domains predicted by SOSUI: None.
(4) CDD domains:
a) KOG0081: GTPase Rab27, small G protein superfamily [Intracellular trafficking, secretion, and vesicular transport].
b) KOG0070: GTP-binding ADP-ribosylation factor Arf1 [Intracellular trafficking, secretion, and vesicular transport].
Motif/Site
(1) Predicted results by ScanProsite:
a) Casein kinase II phosphorylation site : [occurs frequently]
2 - 5: SdgD,
56 - 59: SgpD,
135 - 138: SdlE.
b) ATP/GTP-binding site motif A (P-loop) : [occurs frequently]
16 - 23: GdsgvGKT.
c) N-myristoylation site : [occurs frequently]
19 - 24: GVgkTS,
167 - 172: GTniSQ,
197 - 202: GVvrSN.
d) Protein kinase C phosphorylation site : [occurs frequently]
62 - 64: TgR.
e) N-glycosylation site : [occurs frequently]
133 - 136: NKSD,
166 - 169: NGTN,
169 - 172: NISQ.
(2) Predicted results of subprograms by PSORT II:
a) N-terminal signal peptide: none
b) KDEL ER retention motif in the C-terminus: none
c) ER Membrane Retention Signals: none
d) VAC possible vacuolar targeting motif: none
e) Actinin-type actin-binding motif: type 1: none; type 2: none
f) Prenylation motif: Found CXC motif in the C-terminus: CGC
g) memYQRL transport motif from cell surface to Golgi: none
h) Tyrosines in the tail: none
i) Dileucine motif in the tail: none
3D Model
(1) ModBase entry found, results here.
(2)ModBase Predicted Comparative 3D Structure of P51159 from UCSC Genome Sorter.



From left to right: Front, Top, and Side views of predicted protein.
2D-PAGE
This protein does not exist in the current release of SWISS-2DPAGE.
Computed theoretical MW=24,868Da, pI=5.09 (NP_899058, representative isoform).
FUNCTION
Ontology
a) Biological process: protein transport, vesicle-mediated transport
b) Biological process: small GTPase mediated signal transduction
c) GTP binding
d) GTPase activity
e) Belongs to the small GTPase superfamily (RAB family).
Location
Membrane-bound.
Interaction
[RAB27A effectors]
RAB27A is a tissue-specific Rab that associates with lysosome-related organelles and secretory granules. Molecular activation involves a GDP to GTP exchange. RAB27A and its close paralog RAB27B interact with at least 10 different effectors, tentatively termed as exophilins or Slp/Slac2. Most of the effectors interact with RAB27 via a conserved RAB27-binding domain, which is present in melanophilin (the leaden gene product, also called Slac2-a) and Slp (synaptotagmin-like protein) proteins. The Slp homology domain (SHD) of Slp1~3 and Slac2-a/b (sequences lacking C2 domains, Slac2) specifically and directly binds the GTP-bound form of RAB27A both in vitro and in intact cells. The SHD of Slp1~3 and Slac2 functions as a RAB27A binding domain (Kuroda, et al (2002a)). The N-terminal SHD consists of two conserved alpha-helical regions (SHD1 and SHD2) that are often separated by two zinc finger motifs. SHD1 of Slac2-a/melanophilin alone is both necessary and sufficient for high affinity specific recognition of the GTP-bound form of RAB27A. By contrast, the zinc finger motifs and SHD2 seem to be important for stabilization of the structure of the SHD or higher affinity RAB27A binding (Fukuda, et al (2002a)). In addition, the SHD of Slp5 preferentially interacted with the GTP-bound form of RAB27A and marginally with RAB3A and RAB6A, both in vitro and in intact cells (Kuroda, et al (2002b)). However, Slp4-a interacts with the GDP-bound form of RAB27A (Fukuda, et al (2003a)).Slp2-a controls melanosome distribution in the cell periphery and regulates the morphology of melanocytes. Slp2-a is the most abundantly expressed of the Slp- and Slac2-family proteins in melanocytes and colocalizes with Rab27a on melanosomes. Knockdown of endogenous Slp2-a protein by small-interfering RNAs (siRNAs) markedly reduced the number of melanosomes in the cell periphery of mouse melanocytes ('peripheral dilution') (Kuroda, et al (2004)).
[RAB27A in melanocytes]
RAB27A directly associates in its GTP-bound form with mature melanosomes through its posttranslational C20 geranylgeranyl lipid tail. The RAB27A interacts with myosin-VIIa and myosin-Va via MyRIP (myosin and RAB interacting protein, also called Slac2-c) or melanophilin and mediates melanosome binding to actin. Slac2-a/melanophilin and Slac2-c/MyRIP are linker proteins between RAB27A and myosin Va. Slac2-a directly interacts with RAB27A and myosin Va via its N-terminal region (amino acids 1 to 146) and the middle region (amino acids 241 to 405), respectively (Fukuda, et al (2002b)). Melanophilin is required with RAB27A to recruit myosin Va to melanosomes in melanocytes (Hume, et al). RAB27A binds to the melanosome first and then recruits melanophilin, which in turn recruits myosin-Va. Melanophilin creates this link by binding to RAB27A in a GTP-dependent fashion through its amino terminus, and to myosin-Va through its carboxy terminus (Wu, et al). Melanophilin directly activates the actin-activated ATPase activity of myosin Va and thus its motor activity (Li, et al). Rab3GEP, previously isolated as a GEF for Rab3a, is identified as the non-redundant Rab27a GEF to activate Rab27a in melanocytes (Figueiredo, et al).
GTP-hydrolysis leads to the inactivation of RAB27A and presumably to the dissociation of melanophilin and myosin Va. cAMP stimulates the expression of RAB27A and rapidly increases the interaction of the melanophilin/Slac2-a with actin, allowing the rapid accumulation of melanosomes in the actin-rich region of the dendrite extremities after the action of melanocyte-differentiating agent such as alpha-melanocyte-stimulating hormone (Passeron, et al). Knockdown of Slac2-a caused perinuclear aggregation of melanosomes alone without altering cell shape (Kuroda, et al (2004)). MITF binds to two E-boxes in the proximal region of the Rab27A promoter and stimulates its transcriptional activity, indicating that RAB27A is a new direct transcriptional target of MITF, and link MITF to melanosome transport(Chiaverini, et al).
[RAB27A in RPE]
Melanophilin and myosin Va, both of which are required for normal melanosome distribution in melanocytes, were not required in RPE, despite the association of myosin Va with the RPE melanosome fraction (Gibbs, et al). A melanophilin homolog, MyRIP, mediates the formation of a tripartite complex with RAB27A and myosin VIIa in retinal pigment epithelium (RPE), to regulate the melanosome motility (El-Amraoui, et al; Futter, et al). However, the RAB27A/MyRIP protein complex does not appear to require recruitment of a myosin on the secretory granules in PC12 cells for function (Waselle, et al). Slac2-c functions as a functional myosin VIIa receptor rather than a myosin Va receptor in melanosome The Rab27A.Slac2-c.myosin VIIa tripartite protein complex regulates the transport of retinal melanosomes in pigment epithelium cells (Kuroda, et al (2005)).
[RAB27A in pancreatic cells]
RAB27A acts in concert with RAB3 interacting proteins in most regulated secretory events (Tolmachova, et al). Granuphilin, whose domain structure is similar to that of the RAB3 effector protein rabphilin3, is localized on the membrane of insulin granules and specifically expressed in pancreatic islets and in pituitary tissue. Granuphilin preferentially binds to the GTP form of RAB27A. The RAB27A/granuphilin complex regulates the exocytosis of insulin-containing dense-core granules (Yi, et al ) through a direct interaction with a t-SNARE plasma membrane syntaxin 1a. Granuphilin directly binds to the H3 domain of syntaxin 1a and plays a role in tethering insulin granules to the plasma membrane by an interaction with both Rab27a and syntaxin 1a (Torii, et al ).In beta-cell line MIN6B1, miR124a increases the levels of SNAP25, Rab3A and Synapsin-1A and decreases those of Rab27A and Noc2. Inhibition of Rab27A expression is mediated by direct binding of miR124a to the 3'-untranslated region of Rab27A mRNA. In contrast, miR96 raises mRNA and protein levels of Granuphilin, a negative modulator of insulin exocytosis, and decreases the expression of Noc2 resulting in a decreased capacity of MIN6B1 cells to respond to secretagogues (Lovis, et al). Actin-bundling protein coronin 3 is a novel Rab27a effector that paradoxically bound guanosine diphosphate (GDP)-Rab27a in the pancreatic beta-cell line MIN6. Coronin 3 directly bound GDP-Rab27a through its beta-propeller structure (Kimura, et al). In contrast to another effector, granuphilin, in beta cells, a Rab27a/b effector, exophilin4/Slp2-a, is specifically expressed in pancreatic alpha cells to promote the targeting of glucagon granules to the plasma membrane (Yu, et al). In insulin exocytosis, granuphilin acts on the granules underneath the plasma membrane, whereas Rab27a acts on those in a more distal area (Kasai, et al ). Rab27a exerts dual roles in glucose-mediated insulin granule exocytosis, facilitating refilling of releasable granule pools while also limiting the rate of release from these pools (Merrins, et al ).
[RAB27A in PC12 cells]
In PC12 cells, three Rab27A effectors (granuphilin, rabphilin and Noc2) exist. Granuphilin-a (also called Slp4-a) is specifically localized on dense-core vesicles in PC12 cells and negatively controls dense-core vesicle exocytosis through specific interaction with Rab27a via the N-terminal Slp homology domain (SHD). Slp4-a simultaneously interacts with Rab27A and Munc18-1 on the dense-core vesicle and with syntaxin-1a in the plasma membrane (Tsuboi, et al ). Bitesize is a granuphilin homolog and the only drosophila synaptotagmin-like protein. Mutations that affect bitesize have reduced cell size and number, resulting in smaller animals that develop slowly (Serano, et al ).Rabphilin and Noc2 interact with Rab3a/b/c/d, Rab8a, and Rab27a/b by cotransfection assay (Fukuda, et al (2003b)). Rabphilin and Noc2 are recruited to dense-core vesicles through specific interaction with Rab27a, not with Rab3a, in PC12 cells. The N-terminal Rab binding domain of rabphilin and Noc2 is proposed to be referred as "RBD27 (Rab binding domain for Rab27)" (Fukuda, et al (2004)).
[RAB27A in platelets]
RAB27 regulates the dense core granule secretion in platelets by employing its binding protein, Munc13-4. Munc13-4 directly bound to GTP-RAB27A and -RAB27B in vitro, but not other GTPases, and enhanced dense core granule secretion (Shirakawa, et al).
[RAB27A in CTLs]
Mutations in Munc13-4 cause familial hemophagocytic lymphohistiocytosis subtype 3 (FHL3), a disease phenotypically related to GS2. Both Rab27a and Munc13-4 are highly expressed in CTLs and mast cells where they colocalize on secretory lysosomes. The region comprising the Munc13 homology domains is essential for the localization of Munc13-4 to secretory lysosomes. The GS2 mutant Rab27a (W73G) strongly reduced binding to Munc13-4, whereas the FHL3 mutant Munc13-4 (Delta 608-611) failed to bind Rab27a. Overexpression of Munc13-4 enhanced degranulation of secretory lysosomes in mast cells, showing that it has a positive regulatory role in secretory lysosome fusion. This observations suggest that the secretion defects seen in GS2 and FHL3 have a common origin, and that the Rab27a/Munc13-4 complex may be an essential regulator of secretory granule fusion with the plasma membrane in hematopoietic cells. Mutations in either of the two genes prevent formation of this complex and abolish secretion (Neeft, et al). Both Slp1 and Slp2-a are expressed and interact with Rab27a in CTL, by forming part of a docking complex, capturing secretory lysosomes at the immunological synapse,contributing to secretory lysosome exocytosis (Holt, et al). The hematopoietic form of Slp2a (Slp2a-hem) is a specific effector of the active form of Rab27a. Rab27a recruits Slp2a-hem on vesicular structures in peripheral CTLs. Following CTL-target cell conjugate formation, the Slp2a-hem/Rab27a complex colocalizes with perforin-containing granules at the immunologic synapse (Menasche, et al (2008)).
[RAB27A in other cells]
(Granulocytes/neutrophils) Rab27a is an essential component of the secretory machinery of azurophilic granules in granulocytes. Rab27a-deficient mice have impaired secretion of MPO (myeloperoxidase) into the plasma in response to lipopolysaccharide. Rab27a and JFC1/Slp1 permit MPO release into the surrounding milieu and constitute key components of the secretory machinery of azurophilic granules in granulocytes (Munafo, et al ). Rab27a is a major component of the exocytic machinery of human neutrophils, modulating the secretion of tertiary and specific granules that are readily mobilized upon neutrophil activation (Herrero-Turrion, et al ). Rab27a effectors JFC1/Slp1 and Munc13-4 are components of the exocytic machinery of granulocytes. Rab27a and JFC1 colocalize in predocked and docked vesicles in granulocytes. JFC1-downregulated granulocytes have impaired MPO secretion. Interference with Rab27a or Munc13-4 but not with JFC1 impaired gelatinase B secretion in neutrophils. Munc13-4 localizes at secretory organelles in neutrophils and plays a central role in the regulation of exocytosis of various sets of secretory organelles. However, mobilization of CD11b was not affected in Munc13-4-deficient neutrophils (Brzezinska, et al ).
(Enothelial cells) Weibel-Palade body-like structures induced in HEK-293 cells by the expression of von Willebrand factor can recruit endogenous RAB27A. In the absence of von Willebrand Factor, RAB27A is not lysosome associated. Newly formed Weibel-Palade bodies lack RAB27A, which is acquired some hours after initial appearance of the cigar-shaped organelle (Hannah, et al). Rab27a and its effector MyRIP both are present on only mature WPB, and this rab/effector complex appears to anchor these WPB to peripheral actin. Depletion of either the Rab or its effector results in a loss of peripheral WPB localization, indicating that this Rab/effector complex controls peripheral distribution and prevents release of incompletely processed WPB content (Nightingale, et al).
(Prostate cells) JFC1 [synaptotagmin-like protein (slp1)], a Rab27a- and PtdIns(3,4,5)P3-binding protein, regulates the androgen-dependent secretion of PSAP and PSA in human LNCaP prostate carcinoma cells. Rab27a and PI3K play a central role in the exocytosis of prostate-specific markers (Johnson, et al ).
(Acinar cells) Rab27 was detected in the apical plasma membrane (APM) and secretory granule membrane (SGM) fractions in acinar cells. Noc2/Rab27 complex is an important constituent of the early stages of IPR-stimulated amylase release (Imai, et al ).
[RAB27B]
RAB27A function can be compensated by a closely related protein, RAB27B. RAB27B is functionally redundant with RAB27A and the pathogenesis of Griscelli syndrome is determined by the relative expression of RAB27A and RAB27B in specialized cell types (Barral, et al ). Up-regulated RAB27B in melanocytes of the Griscelli patient can partially take over the function of Rab27a, which could explain the fact that this patient had an evenly pigmented skin and was able to tan (Westbroek, et al (2004)).
RAB27 shows some homology to Sec4p, which interacts with Myo2b. 8 proteins are shown to be associated with SEC4 in Yeast GRID.
RAB27A drosophila homolog CG14791/Rab27 interaction information in CuraGen interaction database.
Pathway
RAB27A is involved in melanosome transport (view diagram of the RAB27A tripartite protein complex and the dynamics of melanosomes here).
RAB27A is required for regulated secretion (targeting and fusion) in cytotoxic T lymphocytes (Stinchcombe, et al) (view diagram of the blockage of Rab27a in ashen CTLs here).
View the m_RABPathway from BioCarta. RAB27A is required for a late step in granule exocytosis (Haddad, et al ).
MUTATION
Allele or SNP
13 mutations deposited in HGMD.
SNPs deposited in dbSNP.
6 selected allelic examples described in OMIM.
Distribution
10 mutations are described in the muation databases of Retina-International scientific Newsletter (Menasche, et al (2000)). Additional mutational alleles are listed below:
1 mutation (67.5kb deletion, from promoter to exon 5) is described in Anikster, et al (2002)
2 novel mutations (c. 400delAA, c.346C>T) are described in Sanal, et al (2002)
1 mutation (c.51delCT) is described in Aksu, et al (2003)
1 mutation (c.352C>T (Q118X)) is described in Bizario, et al (2004)
2 novel mutations (c.259G>C (A87P), g.20411-48243del) are described in Zur Stadt, et al (2006)
A novel mutation, R200X, is described in Rajadhyax, et al (2007)
A novel missense mutation, c.127G>A (p.G43S), is reported in Westbroek, et al (2008) in an Afghani GS2 patient
4 novel mutations (c.131T>C, c.148delA+c.149G>C, c.340delA, c.514delCAAGC) are reported by Mamishi, et al (2008) in Iran, and the c.514delCAAGC mutation is also reported in a Turkish GS2 patient by Onay, et al (2008) .
Effect
Most of the mutations lead to RAB27A-null mutations. Bahadoran, et al studied the three missense mutations (W73G, L130P, and A152P) to show the consequences of the mutations and Menasche, et al (2003) identified the W73 residue of Rab27a as a key position for interaction with the specific effectors of Rab27a. The genotype-phenotype relationships of the eight RAB27A mutations were described in Sanal, et al. The missense mutations Ala87Pro in Rab27a and Leu403Pro in hMunc13-4 each prevented the formation of a stable hMunc13-4/Rab27a complex in vitro (Zur Stadt, et al). Rab27A (G43S) fails to interact with its effector Melanophilin, indicating that the switch I region functions in the recruitment of Rab effector proteins (Westbroek, et al (2008)) .
PHENOTYPE
Defects in RAB27A (OMIM:603868) are a cause of Griscelli syndrome type 2 (GS2) (OMIM:607624) (Menasche, et al (2000)). Griscelli syndrome is a rare autosomal recessive disorder that results in pigmentary dilution of the skin and hair, the presence of large clumps of pigment in hair shafts, and an accumulation of melanosomes in melanocytes. GS2 patients also develop an uncontrolled T lymphocyte and macrophage activation syndrome, known as hemophagocytic syndrome (HS), leading to death in the absence of bone marrow transplantation. GS2 T cells exhibited reduced cytoxicity and cytolytic granule exocytosis, whereas GS1 T cells did not (Menasche, et al (2000); Stinchcombe, et al). CTLs in a GS2 patient are not functional and NK cytotoxicity against K562 or 721.221 cells is diminished (Gazit, et al). Neurological impairment is present in some patients, likely as a result of hemophagocytic syndrome (Sanal, et al ). Rajadhyax, et al reported a case that presented with obstructive hydrocephalus and infiltrative lesions in the brain unaccompanied by other features of accelerated phase.
REFERENCE
- Aksu G, Kutukculer N, Genel F, Vergin C, Omowaire B. Griscelli syndrome without hemophagocytosis in an eleven-year-old girl: expanding the phenotypic spectrum of Rab27A mutations in humans. Am J Med Genet 2003; 116A: 329-33. PMID: 12522785
- Anikster Y, Huizing M, Anderson PD, Fitzpatrick DL, Klar A, Gross-Kieselstein E, Berkun Y, Shazberg G, Gahl WA, Hurvitz H. Evidence that Griscelli syndrome with neurological involvement is caused by mutations in RAB27A, not MYO5A. Am J Hum Genet 2002; 71: 407-14. PMID: 12058346
- Bahadoran P, Busca R, Chiaverini C, Westbroek W, Lambert J, Bille K, Valony G, Fukuda M, Naeyaert JM, Ortonne JP, Ballotti R. Characterization of the molecular defects in Rab27a, caused by RAB27A missense mutations found in patients with Griscelli syndrome. J Biol Chem 2003; 278: 11386-92. PMID: 12531900
- Barral DC, Ramalho JS, Anders R, Hume AN, Knapton HJ, Tolmachova T, Collinson LM, Goulding D, Authi KS, Seabra MC. Functional redundancy of Rab27 proteins and the pathogenesis of Griscelli syndrome. J Clin Invest 2002; 110: 247-57. PMID: 12122117
- Bizario JC, Feldmann J, Castro FA, Menasche G, Jacob CM, Cristofani L, Casella EB, Voltarelli JC, Saint-Basile GD, Espreafico EM. Griscelli Syndrome: Characterization of a New Mutation and Rescue of T-cytotoxic Activity by Retroviral Transfer of RAB27A Gene. J Clin Immunol 2004; 24: 397-410. PMID: 15163896
- Brzezinska AA, Johnson JL, Munafo DB, Crozat K, Beutler B, Kiosses WB, Ellis BA, Catz SD. The Rab27a Effectors JFC1/Slp1 and Munc13-4 Regulate Exocytosis of Neutrophil Granules. Traffic 2008; [Epub ahead of print] PMID: 18939952
- Chiaverini C, Beuret L, Flori E, Busca R, Abbe P, Bille K, Bahadoran P, Ortonne JP, Bertolotto C, Ballotti R. Microphthalmia associated transcription factor regulates rab27A gene expression and controls melanosomes transport. J Biol Chem 2008; 283: 12635-42. PMID: 18281284
- El-Amraoui A, Schonn JS, Kussel-Andermann P, Blanchard S, Desnos C, Henry JP, Wolfrum U, Darchen F, Petit C. MyRIP, a novel Rab effector, enables myosin VIIa recruitment to retinal melanosomes. EMBO Rep 2002; 3: 463-70. PMID: 11964381
- Figueiredo AC, Wasmeier C, Tarafder AK, Ramalho JS, Baron RA, Seabra MC. Rab3GEP is the non-redundant guanine nucleotide exchange factor for Rab27a in melanocytes. J Biol Chem 2008; 283: 23209-16.PMID: 18559336
- Fukuda M, Kanno E, Yamamoto A. Rabphilin and Noc2 are recruited to dense-core vesicles through specific interaction with Rab27A in PC12 cells. J Biol Chem 2004; 279: 13065-75. PMID: 14722103
- Fukuda M. Slp4-a/granuphilin-a inhibits dense-core vesicle exocytosis through interaction with the GDP-bound form of Rab27A in PC12 cells. J Biol Chem 2003a; 278: 15390-6. PMID: 12590134
- Fukuda M. Distinct Rab binding specificity of Rim1, Rim2, rabphilin, and Noc2. Identification of a critical determinant of Rab3A/Rab27A recognition by Rim2. J Biol Chem 2003b; 278: 15373-80. PMID: 12578829
- Fukuda M. Synaptotagmin-like protein (Slp) homology domain 1 of Slac2-a/melanophilin is a critical determinant of GTP-dependent specific binding to Rab27A. J Biol Chem 2002a; 277: 40118-24. PMID: 12189142
- Fukuda M, Kuroda TS, Mikoshiba K. Slac2-a/melanophilin, the missing link between Rab27 and myosin Va: implications of a tripartite protein complex for melanosome transport. J Biol Chem 2002b; 277: 12432-6. PMID: 11856727
- Futter CE, Ramalho JS, Jaissle GB, Seeliger MW, Seabra MC. The role of Rab27a in the regulation of melanosome distribution within retinal pigment epithelial cells. Mol Biol Cell 2004; 15: 2264-75. PMID: 14978221
- Gibbs D, Azarian SM, Lillo C, Kitamoto J, Klomp AE, Steel KP, Libby RT, Williams DS. Role of myosin VIIa and Rab27a in the motility and localization of RPE melanosomes. J Cell Sci 2004; 117: 6473-83.PMID: 15572405
- Haddad EK, Wu X, Hammer JA 3rd, Henkart PA. Defective granule exocytosis in Rab27a-deficient lymphocytes from Ashen mice. J Cell Biol 2001; 152: 835-42. PMID: 11266473
- Hannah MJ, Hume AN, Arribas M, Williams R, Hewlett LJ, Seabra MC, Cutler DF. Weibel-Palade bodies recruit Rab27 by a content-driven, maturation-dependent mechanism that is independent of cell type. J Cell Sci 2003; 116: 3939-48. PMID: 12928333
- Herrero-Turrion MJ, Calafat J, Janssen H, Fukuda M, Mollinedo F. Rab27a regulates exocytosis of tertiary and specific granules in human neutrophils. J Immunol 2008; 181: 3793-803. PMID: 18768832
- Holt O, Kanno E, Bossi G, Booth S, Daniele T, Santoro A, Arico M, Saegusa C, Fukuda M, Griffiths GM. Slp1 and Slp2-a Localize to the Plasma Membrane of CTL and Contribute to Secretion from the Immunological Synapse. Traffic 2008; 9: 446-57. PMID: 18266782
- Hume AN, Collinson LM, Hopkins CR, Strom M, Barral DC, Bossi G, Griffiths GM, Seabra MC. The leaden gene product is required with Rab27a to recruit myosin Va to melanosomes in melanocytes. Traffic 2002; 3: 193-202. PMID: 11886590
- Imai A, Yoshie S, Nashida T, Shimomura H, Fukuda M. Functional involvement of Noc2, a Rab27 effector, in rat parotid acinar cells. Arch Biochem Biophys. 2006; 455: 127-35. PMID: 17067543
- Johnson JL, Ellis BA, Noack D, Seabra MC, Catz SD. The Rab27a-binding protein, JFC1, regulates androgen-dependent secretion of prostate-specific antigen and prostatic-specific acid phosphatase. Biochem J 2005; 391: 699-710. PMID: 16004602
- Kasai K, Fujita T, Gomi H, Izumi T. Docking is not a prerequisite but a temporal constraint for fusion of secretory granules. Traffic 2008; 9: 1191-203.PMID: 18397364
- Kimura T, Kaneko Y, Yamada S, Ishihara H, Senda T, Iwamatsu A, Niki I. The GDP-dependent Rab27a effector coronin 3 controls endocytosis of secretory membrane in insulin-secreting cell lines. J Cell Sci 2008; 121: 3092-8.PMID: 18768935
- Kuroda TS, Fukuda M, Ariga H, Mikoshiba K. The Slp homology domain of synaptotagmin-like proteins 1-4 and Slac2 functions as a novel Rab27A binding domain. J Biol Chem 2002a; 277: 9212-8. PMID: 11773082
- Kuroda TS, Fukuda M, Ariga H, Mikoshiba K. Synaptotagmin-like protein 5: a novel Rab27A effector with C-terminal tandem C2 domains. Biochem Biophys Res Commun 2002b; 293: 899-906. PMID: 12051743
- Kuroda TS, Fukuda M.Rab27A-binding protein Slp2-a is required for peripheral melanosome distribution and elongated cell shape in melanocytes. Nat Cell Biol 2004; 6: 1195-203.PMID: 15543135
- Kuroda TS, Fukuda M. Functional analysis of Slac2-c/MyRIP as a linker protein between melanosomes and myosin VIIa. J Biol Chem. 2005; 280: 28015-22.PMID: 15927964
- Li XD, Ikebe R, Ikebe M. Activation of Myosin Va function by melanophilin, a specific docking partner of Myosin Va. J Biol Chem 2005; 280:17815-22. PMID: 15760894
- Lovis P, Gattesco S, Regazzi R. Regulation of the expression of components of the machinery of exocytosis of insulin-secreting cells by microRNAs. Biol Chem 2008; 389: 305-12. PMID: 18177263
- Mamishi S, Modarressi MH, Pourakbari B, Tamizifar B, Mahjoub F, Fahimzad A, Alyasin S, Bemanian MH, Hamidiyeh AA, Fazlollahi MR, Ashrafi MR, Isaeian A, Khotaei G, Yeganeh M, Parvaneh N. Analysis of RAB27A Gene in Griscelli Syndrome type 2: Novel Mutations Including a Deletion Hotspot. J Clin Immunol 2008; 28: 384-9. PMID: 18350256
- Menasche G, Feldmann J, Houdusse A, Desaymard C, Fischer A, Goud B, de Saint Basile G. Biochemical and functional characterization of Rab27a mutations occurring in Griscelli syndrome patients. Blood 2003; 101: 2736-42. PMID: 12446441
- Menasche G, Menager MM, Lefebvre JM, Deutsch E, Athman R, Lambert N, Mahlaoui N, Court M, Garin J, Fischer A, de Saint Basile G. A newly identified isoform of Slp2a associates with Rab27a in cytotoxic T cells and participates to cytotoxic granule secretion. Blood 2008; 112: 5052-62. PMID: 18812475
- Menasche G, Pastural E, Feldmann J, Certain S, Ersoy F, Dupuis S, Wulffraat N, Bianchi D, Fischer A, Le Deist F, de Saint Basile G. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat Genet 2000; 25: 173-6. PMID: 10835631
- Merrins MJ, Stuenkel EL. Kinetics of Rab27a-dependent actions on vesicle docking and priming in pancreatic beta-cells. J Physiol 2008; 586: 5367-81. PMID: 18801842
- Munafo DB, Johnson JL, Ellis BA, Rutschmann S, Beutler B, Catz SD. Rab27a is a key component of the secretory machinery of azurophilic granules in granulocytes. Biochem J 2007; 402: 229-39.PMID: 17090228
- Neeft M, Wieffer M, de Jong AS, Negroiu G, Metz CH, van Loon A, Griffith J, Krijgsveld J, Wulffraat N, Koch H, Heck AJ, Brose N, Kleijmeer M, van der Sluijs P. Munc13-4 is an effector of rab27a and controls secretion of lysosomes in hematopoietic cells. Mol Biol Cell 2005; 16:731-41. PMID: 15548590
- Nightingale TD, Pattni K, Hume AN, Seabra MC, Cutler DF. Rab27a and MyRIP regulate the amount and multimeric state of VWF released from endothelial cells. Blood 2009; [Epub ahead of print] PMID: 19270261
- Onay H, Balkan C, Cogulu O, Aydinok Y, Karapinar DY, Ozkinay F. A further Turkish case of Griscelli syndrome with new RAB27A mutation. J Am Acad Dermatol 2008; 58: S115-6. No abstract available.
- Passeron T, Bahadoran P, Bertolotto C, Chiaverini C, Busca R, Valony G, Bille K, Ortonne JP, Ballotti R. Cyclic AMP promotes a peripheral distribution of melanosomes and stimulates melanophilin/Slac2-a and actin association. FASEB J 2004; 18: 989-91. PMID: 15059972
- Rajadhyax M, Neti G, Crow Y, Tyagi A. Neurological presentation of Griscelli syndrome: obstructive hydrocephalus without haematological abnormalities or organomegaly. Brain Dev 2007; 29: 247-50. PMID: 17085000
- Sanal O, Ersoy F, Tezcan I, Metin A, Yel L, Menasche G, Gurgey A, Berkel I, de Saint Basile G. Griscelli disease: genotype-phenotype correlation in an array of clinical heterogeneity. J Clin Immunol 2002; 22: 237-43. PMID: 12148598
- Serano J, Rubin GM. The Drosophila synaptotagmin-like protein bitesize is required for growth and has mRNA localization sequences within its open reading frame. Proc Natl Acad Sci U S A 2003; 100: 13368-73. PMID: 14581614
- Shirakawa R, Higashi T, Tabuchi A, Yoshioka A, Nishioka H, Fukuda M, Kita T, Horiuchi H. Munc13-4 is a GTP-Rab27-binding protein regulating dense core granule secretion in platelets. J Biol Chem 2004; 279: 10730-7. PMID: 14699162
- Stinchcombe JC, Barral DC, Mules EH, Booth S, Hume AN, Machesky LM, Seabra MC, Griffiths GM. Rab27a is required for regulated secretion in cytotoxic T lymphocytes. J Cell Biol 2001; 152: 825-34. PMID: 11266472
- Tolmachova T, Anders R, Stinchcombe J, Bossi G, Griffiths GM, Huxley C, Seabra MC. A general role for Rab27a in secretory cells. Mol Biol Cell 2004; 15: 332-44. PMID: 14617806
- Torii S, Takeuchi T, Nagamatsu S, Izumi T. Rab27 effector granuphilin promotes the plasma membrane targeting of insulin granules via interaction with syntaxin 1a. J Biol Chem 2004; 279: 22532-8. PMID: 15028737
- Tsuboi T, Fukuda M. The Slp4-a Linker Domain Controls Exocytosis through Interaction with Munc18-1.Syntaxin-1a Complex. Mol Biol Cell 2006; [Epub ahead of print] PMID: 16481396
- Waselle L, Coppola T, Fukuda M, Iezzi M, El-Amraoui A, Petit C, Regazzi R. Involvement of the Rab27 binding protein Slac2c/MyRIP in insulin exocytosis. Mol Biol Cell 2003; 14: 4103-13. PMID: 14517322
- Westbroek W, Lambert J, De Schepper S, Kleta R, Van Den Bossche K, Seabra MC, Huizing M, Mommaas M, Naeyaert JM. Rab27b is up-regulated in human Griscelli syndrome type II melanocytes and linked to the actin cytoskeleton via exon F-Myosin Va transcripts. Pigment Cell Res 2004; 17: 498-505.PMID: 15357836
- Westbroek W, Tuchman M, Tinloy B, De Wever O, Vilboux T, Hertz JM, Hasle H, Heilmann C, Helip-Wooley A, Kleta R, Gahl WA. A novel missense mutation (G43S) in the switch I region of Rab27A causing Griscelli syndrome. Mol Genet Metab 2008; 94: 248-54. PMID: 18397837
- Wu XS, Rao K, Zhang H, Wang F, Sellers JR, Matesic LE, Copeland NG, Jenkins NA, Hammer JA 3rd. Identification of an organelle receptor for myosin-Va. Nat Cell Biol 2002; 4: 271-8. PMID: 11887186
- Yi Z, Yokota H, Torii S, Aoki T, Hosaka M, Zhao S, Takata K, Takeuchi T, Izumi T. The Rab27a/granuphilin complex regulates the exocytosis of insulin-containing dense-core granules. Mol Cell Biol 2002; 22: 1858-67. PMID: 11865063
- Yu M, Kasai K, Nagashima K, Torii S, Yokota-Hashimoto H, Okamoto K, Takeuchi T, Gomi H, Izumi T. Exophilin4/Slp2-a targets glucagon granules to the plasma membrane through unique Ca2+-inhibitory phospholipid-binding activity of the C2A domain. Mol Biol Cell 2007 ; 18: 688-96. PMID: 17182843
- Zur Stadt U, Beutel K, Kolberg S, Schneppenheim R, Kabisch H, Janka G, Hennies HC. Mutation spectrum in children with primary hemophagocytic lymphohistiocytosis: molecular and functional analyses of PRF1, UNC13D, STX11, and RAB27A. Hum Mutat 2006; 27: 62-8.PMID: 16278825
EDIT HISTORY:
Created by Wei Li & Jonathan Bourne: 07/20/2004
Updated by Wei Li: 06/25/2005
Updated by Wei Li: 04/06/2006
Updated by Wei Li: 02/28/2008
Updated by Wei Li: 08/20/2008
Updated by Wei Li: 03/12/2009