GENOMIC
Mapping
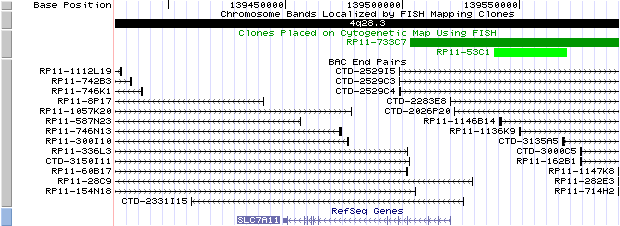
4q28.3. View the map and FISH BAC clones (data from UCSC genome browser).
Structure
(assembly 05/2004)
SLC7A11: 12 exons, 71,737bp , chr4:139,449,092-139,520,828.
The figure below shows the structure of the SLC7A11 gene (data from UCSC genome browser).

Regulatory Element
Search the 5'UTR and 1kb upstream regions (seq1=human SLC7A11, seq2=mouse Slc7a11) by CONREAL with 80% Position Weight Matrices (PWMs) threshold (view results here). Two amino acid response elements (AAREs), each located in the opposite direction with an intervening sequence, were found in the 5'-flanking region of the mouse xCT gene (Sato, et al (2004)). The 5'-deletion analysis revealed that the sequence between -116 and -82 is essential for the basal expression and the sequence between -226 and -116 containing EpRE-1 is essential in response to diethyl maleate(Sasaki, et al).
TRANSCRIPT
RefSeq/ORF
SLC7A11(NM_014331), 3,144bp, view ORF and the alignment to genomic.
Expression Pattern
Tissue specificity: Widely expressed. It is expressed in Muller and retinal pigment epithelial cells, and a retinal ganglion cell line (RGC-5). Oxidative stress upregulates this transport system in RGC-5 cells (Dun, et al). Northern blot analysis demonstrated that the expression of both xCT and 4F2hc was significantly enhanced by oxygen. (Sato, et al (2000)).
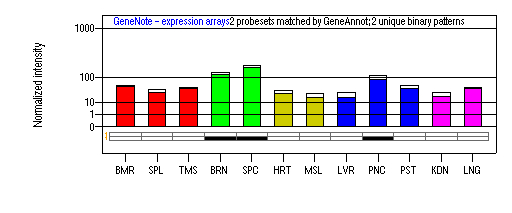
BMR: Bone marrow; SPL: Spleen; TMS: Thymus; BRN: Brain; SPC: Spinal cord; HRT: Heart; MSL: Skeletal muscle; LVR; Liver; PNC: Pancreas; PST: Prostate; KDN: Kidney; LNG: Lung. (data from GeneCards )
PROTEIN
Sequence
xCT (NP_055146): 501aa, UniProtKB/Swiss-Prot entry Q9UPY5.
Synonym: Amino acid transport system xc-, Calcium channel blocker resistance protein CCBR1, Cystine/glutamate transporter, solute carrier family 7 (cationic amino acid transporter, y+ system) member 11
Ortholog
Species
Mouse Rat Dog Fowl GeneView
Slc7a11
Slc7a11
LOC483821
LOC428731
Protein
NP_036120 (502aa)
XP_227120 (502aa)
XP_540941 (551aa)
XP_426289 (580aa)
Identities
89% /500aa 87% /500aa 92% /482aa 68% /578aa
View multiple sequence alignment (PDF file) by ClustalW and GeneDoc.
Domain
(1) Domains predicted by SMART:
AA_permease: 48- 479
(2) Transmembrane domains predicted by SOSUI:
This amino acid sequence is of a MEMBRANE PROTEIN which have 13 transmembrane helices.
No. N terminal transmembrane region C terminal type length
1 46 LLRGVSIIIGTIIGAGIFISPKG 68 PRIMARY 23
2 75 SVGMSLTIWTVCGVLSLFGALSY 97 PRIMARY 23
3 109 GHYTYILEVFGPLPAFVRVWVEL 131 SECONDARY 23
4 136 PAATAVISLAFGRYILEPFFIQC 158 SECONDARY 23
5 162 ELAIKLITAVGITVVMVLNSMSV 184 PRIMARY 23
6 192 IFLTFCKLTAILIIIVPGVMQLI 214 PRIMARY 23
7 235 LPLAFYYGMYAYAGWFYLNFVTE 257 SECONDARY 23
8 268 LAICISMAIVTIGYVLTNVAYFT 290 PRIMARY 23
9 298 LLSNAVAVTFSERLLGNFSLAVP 320 SECONDARY 23
10 363 HTPLPAVIVLHPLTMIMLFSGDL 385 SECONDARY 23
11 389 LNFLSFARWLFIGLAVAGLIYLR 411 PRIMARY 23
12 422 KVPLFIPALFSFTCLFMVALSLY 444 PRIMARY 23
13 450 TGIGFVITLTGVPAYYLFIIWD 471 SECONDARY 22
Note: a topological model for xCT of 12 transmembrane domains with the N and C termini located inside the cell was proposed by Gasel et al .
(3) Graphic view of InterPro domain structure.
Motif/Site
(1) Predicted results by ScanProsite:
a) N-glycosylation site: 314 to 317 NFSL.
b) cAMP- and cGMP-dependent protein kinase phosphorylation site: 42 to 45 RKVT; 361 to 364 RKHT.
c) Protein kinase C phosphorylation site: 65 to 67 SPK; 103 to 105 TIK; 187 to 189 SAR; 226 to 228 SGR; 308 to 310 SER; 481 to 483 SEK.
d) Casein kinase II phosphorylation site: 96 to 99 SYAE; 226 to 229 SGRD; 306 to 309 TFSE.
e) N-myristoylation site: 9 sites.
(2) Predicted results of subprograms by PSORT II:
a) N-terminal signal peptide: none
b) 11 tentative TMs, membrane topology: type 3a
c) KDEL ER retention motif in C-terminus: none
d) ER membrane retention signals:
XXRR-like motif in the N-terminus: VRKP; KKXX-like motif in the C-terminus: EEDK
e) VAC possible vacuolar targeting motif: none
f) Actinin-type actin-binding motif: type 1: none; type 2: none
g) Prenylation motif: none
h) memYQRL transport motif from cell surface to Golgi: none
i) Tyrosines in the tail: none
j) Dileucine motif in the tail: none
3D Model
(1)ModBase predicted comparative 3D structure of Q9UPY5 from UCSC Genome Sorter.
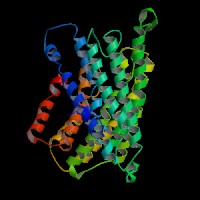
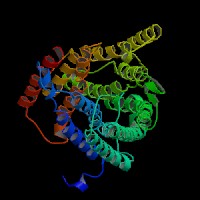
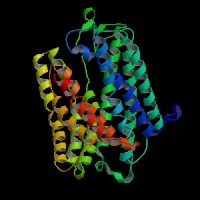
From left to right: Front, Top, and Side views of predicted protein.
2D-PAGE
This protein does not exist in the current release of SWISS-2DPAGE.
Computed theoretical MW=55,423Da, pI=9.29 (NP_055146).
FUNCTION
Ontology
(1) Biological process: amino acid transport.
(2) Pheomelanin production.
(3) Oxidative stress.
(4) Belongs to the amino acid-polyamine-organocation (APC) superfamily. L-type amino acid transporter (LAT) (TC 2.A.3.8) family.
Location
Integral membrane protein. Transport system xc- was found in plasma membrane of cultured mammalian cells (Sato, et al (1999)).
Interaction
SLC7A11 (xCT), together with SLC3A2 (4F2hc, or CD98) linked by a disulfide bond at residue C158, encodes the heterodimeric amino acid transport system x(c)(-), which mediates cystine-glutamate exchange and thereby regulates intracellular glutathione levels. System x(c)- activity is important to maintain both intracellular glutathione levels and the redox balance between cystine and cysteine in the extracellular milieu. rBAT can replace 4F2hc in the expression of the activity of system x(c)(-) (Wang, et al).
In mouse NIH3T3 cells, the activity of system x(c)(-) and xCT mRNA is induced not only by deprivation of cystine but also by deprivation of other amino acids via a genomic cis-element termed amino acid response element (AARE) (Sato, et al (2004)). Overexpression of Nrf-2 in astrocytes specifically increases expression of the xCT cystine/glutamate antiporter and other thiol synthesis-related proteins, to promote cell survival (Qiang, et al). Cys(327) is a functionally important residue accessible to the aqueous extracellular environment and is structurally linked to the permeation pathway and/or the substrate binding site (Jimenez-Vidal, et al).
A CD44 variant (CD44v) interacts with xCT and controls the intracellular level of reduced glutathione (GSH). Ablation of CD44 induced loss of xCT from the cell surface and suppressed tumor growth in a transgenic mouse model of gastric cancer. It also induced activation of p38(MAPK), a downstream target of ROS, and expression of the gene for the cell cycle inhibitor p21(CIP1/WAF1)(Ishimoto, et al). xCT has been identified as a receptor mediating KSHV cell fusion (Kaleeba, et al).
miR-26b, which is down-regulated in human breast cancer specimens and cell lines, impairs viability and triggers apoptosis of human breast cancer MCF7 cells. SLC7A11 is identified as a direct target of miR-26b and its expression is remarkably increased in both breast cancer cell lines and clinical samples (Liu, et al).
Pathway
System x(c)(-) is a sodium-independent, high-affinity transporter of anionic amino acids with high specificity for anionic form of cystine and glutamate. Chintala, et al have reported that Slc7a11 regulates the pheomelanin production and cell proliferation. In melanosomes, dopaquinone reacts with cysteine to yield cysteinldopa, which is a precursor of pheomelanin. Cysteine is a component of the tripeptide glutathione. Glutathione metabolism pathway is described in KEGG Pathways.
MUTATION
Allele or SNP
No mutation has been reported in 32 human HPS patients who lack mutations in the seven known HPS genes (Chintala, et al).
SNPs deposited in dbSNP.
Distribution
(none)
Effect
(none)
PHENOTYPE
SLC7A11 is described in OMIM 607933.
Cystine uptake in human cancer cells is largely mediated by system Xc. xCT is relatively highly expressed in lung, colon, and central nervous system cancer cells (Huang, et al). Modulation of xCT activity is possiblely untilized in cancer therapy. Pharmacologic inhibition of system Xc remarkably inhibits the proliferation of several types of cancer cells, including lymphoma, glioma, prostate and breast cancer (Chung, et al; Doxsee, et al; Gout, et al; Narang, et al). This is thought be mediated by the depletion of GSH, which results in an increase of reactive oxygen species (ROS), and ultimately leads to caspase-dependent apoptosis (Doxsee, et al; Sakakura, et al). The apoptosis is likely mediated by c-Jun N-terminal kinase (JNK) activation. The JNK activation triggers both a caspase-dependent (caspases-9 and -3) and an ER stress-mediated (eIF2 and CHOP) pathway to induce apoptosis (Qiao, et al). In the absence of phospho-eIF2alpha, xCT expression is down-regulated that is mediated by nuclear factor ATF4, implicating the role eIF2alpha-ATF4-xCT module in neurodegeneration (Lewerenz, et al).
Disruption of xCT enhanced homotypic cell-cell adhesion and attenuated cell-extracellular matrix adhesion in an esophageal cancer cell line, KYSE150. SASP significantly inhibited both cell invasion of KYSE150 in vitro and its experimental metastasis in nude mice. Caveolin-1 was upregulated and beta-catenin was recruited to the plasma membrane when xCT was deficient, which were followed by the inhibition of beta-catenin transcriptional activity. Upregulation of caveolin-1 and inhibition of tumor cell invasion were mediated by reactive oxygen species-induced p38 MAPK activation (Chen, et al). Savaskan et al show that genetic or pharmacological inhibition of the glutamate transporter Xc system in gliomas leads to abrogated neurodegeneration, attenuated perifocal edema and prolonged survival. xCT deficiency causes unremitting inflammation because of the impaired survival of activated macrophages at the inflammatory site (Nabeyama, et al).
Kaposi's sarcoma-associated herpesvirus (KSHV, human herpesvirus 8) is the causative agent of Kaposi's sarcoma and other lymphoproliferative syndromes often associated with HIV/AIDS. xCT mediates the entry of KSHV which implicates its involvement in the infectious process of this pathogen (Kaleeba, et al; Veettil, et al (2006); Rappocciolo, et al). KSHV interacts with functionally related integrins (alphaVbeta3, alpha3beta1, and alphaVbeta5) and CD98/xCT molecules in a temporal fashion to form a multimolecular complex during the early stages of endothelial cell infection, probably mediating multiple roles in entry, signal transduction, and viral-gene expression (Veettil, et al (2008)). xCT was overexpressed in KS tissues and HHV-8-positive BCBL-1 cells. 14-3-3beta is a downstream effector of xCT in KS to mediate the cell proliferation (Zeng, et al). KSHV-encoded microRNAs (KSHV miRNAs) upregulate xCT expression by macrophages and endothelial cells, largely through miR-K12-11 suppression of BACH-1-a negative regulator of transcription recognizing antioxidant response elements within gene promoters (Qin, et al).
REFERENCE
- Chen RS, Song YM, Zhou ZY, Tong T, Li Y, Fu M, Guo XL, Dong LJ, He X, Qiao HX, Zhan QM, Li W. Disruption of xCT inhibits cancer cell metastasis via the caveolin-1/beta-catenin pathway. Oncogene 2009; 28: 599-609. PMID: 19015640
- Chintala S, Li W, Lamoreux ML, Ito S, Wakamatsu K, Sviderskaya EV, Bennett DC, Park YM, Gahl WA, Huizing M, Spritz RA, Ben S, Novak EK, Tan J, Swank RT.
Slc7a11 gene controls production of pheomelanin pigment and proliferation of cultured cells. Proc Natl Acad Sci U S A 2005; 102: 10964-9. PMID: 16037214
- Chung WJ, Lyons SA, Nelson GM, Hamza H, Gladson CL, Gillespie GY, Sontheimer H. Inhibition of cystine uptake disrupts the growth of primary brain tumors.
J Neurosci 2005; 25: 7101-10.
PMID: 16079392
- Doxsee DW, Gout PW, Kurita T, Lo M, Buckley AR, Wang Y, Xue H, Karp CM, Cutz JC, Cunha GR, Wang YZ. Sulfasalazine-induced cystine starvation: potential use for prostate cancer therapy. Prostate 2007; 67: 162-71.
PMID: 17075799
- Dun Y, Mysona B, Van Ells T, Amarnath L, Shamsul Ola M, Ganapathy V, Smith SB. Expression of the cystine-glutamate exchanger (x(c) (-)) in retinal ganglion cells and regulation by nitric oxide and oxidative stress. Cell Tissue Res 2006; 324: 189-202. PMID: 16444546
- Gasol E, Jimenez-Vidal M, Chillaron J, Zorzano A, Palacin M. Membrane topology of system xc- light subunit reveals a re-entrant loop with substrate-restricted accessibility.
J Biol Chem 2004; 279: 31228-36. PMID: 15151999
- Gout PW, Buckley AR, Simms CR, Bruchovsky N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the x(c)- cystine transporter: a new action for an old drug. Leukemia. 2001; 15: 1633-40.PMID: 11587223
- Huang Y, Dai Z, Barbacioru C, Sad¨¦e W. Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity and chemoresistance.Cancer Res 2005; 65: 7446-54.
PMID: 16103098
- Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, Masuko T, Shimizu T, Ishikawa T, Kai K, Takahashi E, Imamura Y, Baba Y, Ohmura M, Suematsu M, Baba H, Saya H. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 2011; 19: 387-400. PMID: 21397861
- Jimenez-Vidal M, Gasol E, Zorzano A, Nunes V, Palacin M, Chillaron J. Thiol modification of cysteine 327 in the eighth transmembrane domain of the light subunit xCT of the heteromeric cystine/glutamate antiporter suggests close proximity to the substrate binding site/permeation pathway.
J Biol Chem 2004; 279: 11214-21. PMID: 14722095
- Kaleeba JA, Berger EA. Kaposi's sarcoma-associated herpesvirus fusion-entry receptor: cystine transporter xCT. Science 2006; 311: 1921-4. PMID: 16574866
- Lewerenz J, Maher P. Basal levels of eIF2alpha phosphorylation determine cellular antioxidant status by regulating ATF4 and xCT expression. J Biol Chem 2009; 284: 1106-15. PMID: 19017641
- Liu XX, Li XJ, Zhang B, Liang YJ, Zhou CX, Cao DX, He M, Chen GQ, He JR, Zhao Q.
MicroRNA-26b is underexpressed in human breast cancer and induces cell apoptosis by targeting SLC7A11. FEBS Lett 2011; 585: 1363-7. PMID: 21510944
- Nabeyama A, Kurita A, Asano K, Miyake Y, Yasuda T, Miura I, Nishitai G, Arakawa S, Shimizu S, Wakana S, Yoshida H, Tanaka M. xCT deficiency accelerates chemically induced tumorigenesis. Proc Natl Acad Sci U S A 2010; 107: 6436-41.
PMID: 20308543
- Narang VS, Pauletti GM, Gout PW, Buckley DJ, Buckley AR. Suppression of cystine uptake by sulfasalazine inhibits proliferation of human mammary carcinoma cells.
Anticancer Res 2003; 23: 4571-9.
PMID: 14981898
- Rappocciolo G, Hensler HR, Jais M, Reinhart TA, Pegu A, Jenkins FJ, Rinaldo CR. Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC-SIGN. J Virol 2008; 82: 4793-806.
PMID: 18337571
- Qiang W, Cahill JM, Liu J, Kuang X, Liu N, Scofield VL, Voorhees JR, Reid AJ, Yan M, Lynn WS, Wong PK. Activation of transcription factor Nrf-2 and its downstream targets in response to moloney murine leukemia virus ts1-induced thiol depletion and oxidative stress in astrocytes. J Virol 2004; 78: 11926-38.
PMID: 15479833
- Qiao HX, Hao CJ, Li Y, He X, Chen RS, Cui J, Xu ZH, Li W. JNK activation mediates the apoptosis of xCT-deficient cells. Biochem Biophys Res Commun 2008; 370: 584-8. PMID: 18395005
- Qin Z, Freitas E, Sullivan R, Mohan S, Bacelieri R, Branch D, Romano M, Kearney P, Oates J, Plaisance K, Renne R, Kaleeba J, Parsons C. Upregulation of xCT by KSHV-encoded microRNAs facilitates KSHV dissemination and persistence in an environment of oxidative stress. PLoS Pathog 2010; 6: e1000742. PMID: 20126446
- Sakakura Y, Sato H, Shiiya A, Tamba M, Sagara J, Matsuda M, Okamura N, Makino N, Bannai S.Expression and function of cystine/glutamate transporter in neutrophils. J Leukoc Biol 2007; 81: 974-82. PMID: 17200146
- Sasaki H, Sato H, Kuriyama-Matsumura K, Sato K, Maebara K, Wang H, Tamba M, Itoh K, Yamamoto M, Bannai S. Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J Biol Chem 2002; 277: 44765-71. PMID: 12235164
- Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins.
J Biol Chem 1999; 274:11455-8. PMID: 10206947
- Sato H, Tamba M, Kuriyama-Matsumura K, Okuno S, Bannai S. Molecular cloning and expression of human xCT, the light chain of amino acid transport system xc-.
Antioxid Redox Signal. 2000; 2:665-71. PMID: 11213471
- Sato H, Tamba M, Okuno S, Sato K, Keino-Masu K, Masu M, Bannai S. Distribution of cystine/glutamate exchange transporter, system x(c)-, in the mouse brain.
J Neurosci 2002; 22: 8028-33.PMID: 12223556
- Sato H, Nomura S, Maebara K, Sato K, Tamba M, Bannai S. Transcriptional control of cystine/glutamate transporter gene by amino acid deprivation.
Biochem Biophys Res Commun 2004; 325: 109-16. PMID: 155522208
- Savaskan NE, Heckel A, Hahnen E, Engelhorn T, Doerfler A, Ganslandt O, Nimsky C, Buchfelder M, Ey¨ąpoglu IY. Small interfering RNA-mediated xCT silencing in gliomas inhibits neurodegeneration and alleviates brain edema. Nat Med 2008;14: 629-32.PMID: 18469825
- Veettil MV, Sharma-Walia N, Sadagopan S, Raghu H, Sivakumar R, Naranatt PP, Chandran B. RhoA-GTPase facilitates entry of Kaposi's sarcoma-associated herpesvirus into adherent target cells in a Src-dependent manner.
J Virol 2006; 80: 11432-46. PMID: 17005646
- Veettil MV, Sadagopan S, Sharma-Walia N, Wang FZ, Raghu H, Varga L, Chandran B. Kaposi's sarcoma-associated herpesvirus forms a multimolecular complex of integrins (alphaVbeta5, alphaVbeta3, and alpha3beta1) and CD98-xCT during infection of human dermal microvascular endothelial cells, and CD98-xCT is essential for the postentry stage of infection. J Virol 2008; 82: 12126-44. PMID: 18829766
- Wang H, Tamba M, Kimata M, Sakamoto K, Bannai S, Sato H. Expression of the activity of cystine/glutamate exchange transporter, system x(c)(-), by xCT and rBAT.
Biochem Biophys Res Commun 2003; 305: 611-8. PMID: 12763038
- Zeng Y, Li Y, Chen RS, He X, Yang L, Li W. Overexpression of xCT induces up-regulation of 14-3-3beta in Kaposi's sarcoma. Biosci Rep 2010; 30: 277-83. PMID: 20100173
EDIT HISTORY:
Created by Wei Li, 08/18/2005
Updated by Wei Li, 04/06/2006
Updated by Wei Li, 12/28/2006
Updated by Wei Li, 08/20/2008
Updated by Wei Li, 03/12/2009
Updated by Wei Li, 05/29/2011
4q28.3. View the map and FISH BAC clones (data from UCSC genome browser).

Structure
(assembly 05/2004)
SLC7A11: 12 exons, 71,737bp , chr4:139,449,092-139,520,828.
The figure below shows the structure of the SLC7A11 gene (data from UCSC genome browser).

Regulatory Element
Search the 5'UTR and 1kb upstream regions (seq1=human SLC7A11, seq2=mouse Slc7a11) by CONREAL with 80% Position Weight Matrices (PWMs) threshold (view results here). Two amino acid response elements (AAREs), each located in the opposite direction with an intervening sequence, were found in the 5'-flanking region of the mouse xCT gene (Sato, et al (2004)). The 5'-deletion analysis revealed that the sequence between -116 and -82 is essential for the basal expression and the sequence between -226 and -116 containing EpRE-1 is essential in response to diethyl maleate(Sasaki, et al).
TRANSCRIPT
RefSeq/ORF
SLC7A11(NM_014331), 3,144bp, view ORF and the alignment to genomic.
Expression Pattern
Tissue specificity: Widely expressed. It is expressed in Muller and retinal pigment epithelial cells, and a retinal ganglion cell line (RGC-5). Oxidative stress upregulates this transport system in RGC-5 cells (Dun, et al). Northern blot analysis demonstrated that the expression of both xCT and 4F2hc was significantly enhanced by oxygen. (Sato, et al (2000)).

BMR: Bone marrow; SPL: Spleen; TMS: Thymus; BRN: Brain; SPC: Spinal cord; HRT: Heart; MSL: Skeletal muscle; LVR; Liver; PNC: Pancreas; PST: Prostate; KDN: Kidney; LNG: Lung. (data from GeneCards )
PROTEIN
Sequence
xCT (NP_055146): 501aa, UniProtKB/Swiss-Prot entry Q9UPY5.
Synonym: Amino acid transport system xc-, Calcium channel blocker resistance protein CCBR1, Cystine/glutamate transporter, solute carrier family 7 (cationic amino acid transporter, y+ system) member 11
Ortholog
Species
Mouse Rat Dog Fowl GeneView
Slc7a11
Slc7a11
LOC483821
LOC428731
Protein
NP_036120 (502aa)
XP_227120 (502aa)
XP_540941 (551aa)
XP_426289 (580aa)
Identities
89% /500aa 87% /500aa 92% /482aa 68% /578aa
View multiple sequence alignment (PDF file) by ClustalW and GeneDoc.
Domain
(1) Domains predicted by SMART:
AA_permease: 48- 479
(2) Transmembrane domains predicted by SOSUI:
This amino acid sequence is of a MEMBRANE PROTEIN which have 13 transmembrane helices.
No. N terminal transmembrane region C terminal type length
1 46 LLRGVSIIIGTIIGAGIFISPKG 68 PRIMARY 23
2 75 SVGMSLTIWTVCGVLSLFGALSY 97 PRIMARY 23
3 109 GHYTYILEVFGPLPAFVRVWVEL 131 SECONDARY 23
4 136 PAATAVISLAFGRYILEPFFIQC 158 SECONDARY 23
5 162 ELAIKLITAVGITVVMVLNSMSV 184 PRIMARY 23
6 192 IFLTFCKLTAILIIIVPGVMQLI 214 PRIMARY 23
7 235 LPLAFYYGMYAYAGWFYLNFVTE 257 SECONDARY 23
8 268 LAICISMAIVTIGYVLTNVAYFT 290 PRIMARY 23
9 298 LLSNAVAVTFSERLLGNFSLAVP 320 SECONDARY 23
10 363 HTPLPAVIVLHPLTMIMLFSGDL 385 SECONDARY 23
11 389 LNFLSFARWLFIGLAVAGLIYLR 411 PRIMARY 23
12 422 KVPLFIPALFSFTCLFMVALSLY 444 PRIMARY 23
13 450 TGIGFVITLTGVPAYYLFIIWD 471 SECONDARY 22
Note: a topological model for xCT of 12 transmembrane domains with the N and C termini located inside the cell was proposed by Gasel et al .
(3) Graphic view of InterPro domain structure.
Motif/Site
(1) Predicted results by ScanProsite:
a) N-glycosylation site: 314 to 317 NFSL.
b) cAMP- and cGMP-dependent protein kinase phosphorylation site: 42 to 45 RKVT; 361 to 364 RKHT.
c) Protein kinase C phosphorylation site: 65 to 67 SPK; 103 to 105 TIK; 187 to 189 SAR; 226 to 228 SGR; 308 to 310 SER; 481 to 483 SEK.
d) Casein kinase II phosphorylation site: 96 to 99 SYAE; 226 to 229 SGRD; 306 to 309 TFSE.
e) N-myristoylation site: 9 sites.
(2) Predicted results of subprograms by PSORT II:
a) N-terminal signal peptide: none
b) 11 tentative TMs, membrane topology: type 3a
c) KDEL ER retention motif in C-terminus: none
d) ER membrane retention signals:
XXRR-like motif in the N-terminus: VRKP; KKXX-like motif in the C-terminus: EEDK
e) VAC possible vacuolar targeting motif: none
f) Actinin-type actin-binding motif: type 1: none; type 2: none
g) Prenylation motif: none
h) memYQRL transport motif from cell surface to Golgi: none
i) Tyrosines in the tail: none
j) Dileucine motif in the tail: none
3D Model
(1)ModBase predicted comparative 3D structure of Q9UPY5 from UCSC Genome Sorter.



From left to right: Front, Top, and Side views of predicted protein.
2D-PAGE
This protein does not exist in the current release of SWISS-2DPAGE.
Computed theoretical MW=55,423Da, pI=9.29 (NP_055146).
FUNCTION
Ontology
(1) Biological process: amino acid transport.
(2) Pheomelanin production.
(3) Oxidative stress.
(4) Belongs to the amino acid-polyamine-organocation (APC) superfamily. L-type amino acid transporter (LAT) (TC 2.A.3.8) family.
Location
Integral membrane protein. Transport system xc- was found in plasma membrane of cultured mammalian cells (Sato, et al (1999)).
Interaction
SLC7A11 (xCT), together with SLC3A2 (4F2hc, or CD98) linked by a disulfide bond at residue C158, encodes the heterodimeric amino acid transport system x(c)(-), which mediates cystine-glutamate exchange and thereby regulates intracellular glutathione levels. System x(c)- activity is important to maintain both intracellular glutathione levels and the redox balance between cystine and cysteine in the extracellular milieu. rBAT can replace 4F2hc in the expression of the activity of system x(c)(-) (Wang, et al).
In mouse NIH3T3 cells, the activity of system x(c)(-) and xCT mRNA is induced not only by deprivation of cystine but also by deprivation of other amino acids via a genomic cis-element termed amino acid response element (AARE) (Sato, et al (2004)). Overexpression of Nrf-2 in astrocytes specifically increases expression of the xCT cystine/glutamate antiporter and other thiol synthesis-related proteins, to promote cell survival (Qiang, et al). Cys(327) is a functionally important residue accessible to the aqueous extracellular environment and is structurally linked to the permeation pathway and/or the substrate binding site (Jimenez-Vidal, et al).
A CD44 variant (CD44v) interacts with xCT and controls the intracellular level of reduced glutathione (GSH). Ablation of CD44 induced loss of xCT from the cell surface and suppressed tumor growth in a transgenic mouse model of gastric cancer. It also induced activation of p38(MAPK), a downstream target of ROS, and expression of the gene for the cell cycle inhibitor p21(CIP1/WAF1)(Ishimoto, et al). xCT has been identified as a receptor mediating KSHV cell fusion (Kaleeba, et al).
miR-26b, which is down-regulated in human breast cancer specimens and cell lines, impairs viability and triggers apoptosis of human breast cancer MCF7 cells. SLC7A11 is identified as a direct target of miR-26b and its expression is remarkably increased in both breast cancer cell lines and clinical samples (Liu, et al).
Pathway
System x(c)(-) is a sodium-independent, high-affinity transporter of anionic amino acids with high specificity for anionic form of cystine and glutamate. Chintala, et al have reported that Slc7a11 regulates the pheomelanin production and cell proliferation. In melanosomes, dopaquinone reacts with cysteine to yield cysteinldopa, which is a precursor of pheomelanin. Cysteine is a component of the tripeptide glutathione. Glutathione metabolism pathway is described in KEGG Pathways.
MUTATION
Allele or SNP
No mutation has been reported in 32 human HPS patients who lack mutations in the seven known HPS genes (Chintala, et al).
SNPs deposited in dbSNP.
Distribution
(none)
Effect
(none)
PHENOTYPE
SLC7A11 is described in OMIM 607933.
Cystine uptake in human cancer cells is largely mediated by system Xc. xCT is relatively highly expressed in lung, colon, and central nervous system cancer cells (Huang, et al). Modulation of xCT activity is possiblely untilized in cancer therapy. Pharmacologic inhibition of system Xc remarkably inhibits the proliferation of several types of cancer cells, including lymphoma, glioma, prostate and breast cancer (Chung, et al; Doxsee, et al; Gout, et al; Narang, et al). This is thought be mediated by the depletion of GSH, which results in an increase of reactive oxygen species (ROS), and ultimately leads to caspase-dependent apoptosis (Doxsee, et al; Sakakura, et al). The apoptosis is likely mediated by c-Jun N-terminal kinase (JNK) activation. The JNK activation triggers both a caspase-dependent (caspases-9 and -3) and an ER stress-mediated (eIF2 and CHOP) pathway to induce apoptosis (Qiao, et al). In the absence of phospho-eIF2alpha, xCT expression is down-regulated that is mediated by nuclear factor ATF4, implicating the role eIF2alpha-ATF4-xCT module in neurodegeneration (Lewerenz, et al).
Disruption of xCT enhanced homotypic cell-cell adhesion and attenuated cell-extracellular matrix adhesion in an esophageal cancer cell line, KYSE150. SASP significantly inhibited both cell invasion of KYSE150 in vitro and its experimental metastasis in nude mice. Caveolin-1 was upregulated and beta-catenin was recruited to the plasma membrane when xCT was deficient, which were followed by the inhibition of beta-catenin transcriptional activity. Upregulation of caveolin-1 and inhibition of tumor cell invasion were mediated by reactive oxygen species-induced p38 MAPK activation (Chen, et al). Savaskan et al show that genetic or pharmacological inhibition of the glutamate transporter Xc system in gliomas leads to abrogated neurodegeneration, attenuated perifocal edema and prolonged survival. xCT deficiency causes unremitting inflammation because of the impaired survival of activated macrophages at the inflammatory site (Nabeyama, et al).
Kaposi's sarcoma-associated herpesvirus (KSHV, human herpesvirus 8) is the causative agent of Kaposi's sarcoma and other lymphoproliferative syndromes often associated with HIV/AIDS. xCT mediates the entry of KSHV which implicates its involvement in the infectious process of this pathogen (Kaleeba, et al; Veettil, et al (2006); Rappocciolo, et al). KSHV interacts with functionally related integrins (alphaVbeta3, alpha3beta1, and alphaVbeta5) and CD98/xCT molecules in a temporal fashion to form a multimolecular complex during the early stages of endothelial cell infection, probably mediating multiple roles in entry, signal transduction, and viral-gene expression (Veettil, et al (2008)). xCT was overexpressed in KS tissues and HHV-8-positive BCBL-1 cells. 14-3-3beta is a downstream effector of xCT in KS to mediate the cell proliferation (Zeng, et al). KSHV-encoded microRNAs (KSHV miRNAs) upregulate xCT expression by macrophages and endothelial cells, largely through miR-K12-11 suppression of BACH-1-a negative regulator of transcription recognizing antioxidant response elements within gene promoters (Qin, et al).
REFERENCE
- Chen RS, Song YM, Zhou ZY, Tong T, Li Y, Fu M, Guo XL, Dong LJ, He X, Qiao HX, Zhan QM, Li W. Disruption of xCT inhibits cancer cell metastasis via the caveolin-1/beta-catenin pathway. Oncogene 2009; 28: 599-609. PMID: 19015640
- Chintala S, Li W, Lamoreux ML, Ito S, Wakamatsu K, Sviderskaya EV, Bennett DC, Park YM, Gahl WA, Huizing M, Spritz RA, Ben S, Novak EK, Tan J, Swank RT.
Slc7a11 gene controls production of pheomelanin pigment and proliferation of cultured cells. Proc Natl Acad Sci U S A 2005; 102: 10964-9. PMID: 16037214
- Chung WJ, Lyons SA, Nelson GM, Hamza H, Gladson CL, Gillespie GY, Sontheimer H. Inhibition of cystine uptake disrupts the growth of primary brain tumors.
J Neurosci 2005; 25: 7101-10.
PMID: 16079392
- Doxsee DW, Gout PW, Kurita T, Lo M, Buckley AR, Wang Y, Xue H, Karp CM, Cutz JC, Cunha GR, Wang YZ. Sulfasalazine-induced cystine starvation: potential use for prostate cancer therapy. Prostate 2007; 67: 162-71.
PMID: 17075799
- Dun Y, Mysona B, Van Ells T, Amarnath L, Shamsul Ola M, Ganapathy V, Smith SB. Expression of the cystine-glutamate exchanger (x(c) (-)) in retinal ganglion cells and regulation by nitric oxide and oxidative stress. Cell Tissue Res 2006; 324: 189-202. PMID: 16444546
- Gasol E, Jimenez-Vidal M, Chillaron J, Zorzano A, Palacin M. Membrane topology of system xc- light subunit reveals a re-entrant loop with substrate-restricted accessibility.
J Biol Chem 2004; 279: 31228-36. PMID: 15151999
- Gout PW, Buckley AR, Simms CR, Bruchovsky N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the x(c)- cystine transporter: a new action for an old drug. Leukemia. 2001; 15: 1633-40.PMID: 11587223
- Huang Y, Dai Z, Barbacioru C, Sad¨¦e W. Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity and chemoresistance.Cancer Res 2005; 65: 7446-54.
PMID: 16103098
- Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, Masuko T, Shimizu T, Ishikawa T, Kai K, Takahashi E, Imamura Y, Baba Y, Ohmura M, Suematsu M, Baba H, Saya H. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 2011; 19: 387-400. PMID: 21397861
- Jimenez-Vidal M, Gasol E, Zorzano A, Nunes V, Palacin M, Chillaron J. Thiol modification of cysteine 327 in the eighth transmembrane domain of the light subunit xCT of the heteromeric cystine/glutamate antiporter suggests close proximity to the substrate binding site/permeation pathway.
J Biol Chem 2004; 279: 11214-21. PMID: 14722095
- Kaleeba JA, Berger EA. Kaposi's sarcoma-associated herpesvirus fusion-entry receptor: cystine transporter xCT. Science 2006; 311: 1921-4. PMID: 16574866
- Lewerenz J, Maher P. Basal levels of eIF2alpha phosphorylation determine cellular antioxidant status by regulating ATF4 and xCT expression. J Biol Chem 2009; 284: 1106-15. PMID: 19017641
- Liu XX, Li XJ, Zhang B, Liang YJ, Zhou CX, Cao DX, He M, Chen GQ, He JR, Zhao Q.
MicroRNA-26b is underexpressed in human breast cancer and induces cell apoptosis by targeting SLC7A11. FEBS Lett 2011; 585: 1363-7. PMID: 21510944
- Nabeyama A, Kurita A, Asano K, Miyake Y, Yasuda T, Miura I, Nishitai G, Arakawa S, Shimizu S, Wakana S, Yoshida H, Tanaka M. xCT deficiency accelerates chemically induced tumorigenesis. Proc Natl Acad Sci U S A 2010; 107: 6436-41.
PMID: 20308543
- Narang VS, Pauletti GM, Gout PW, Buckley DJ, Buckley AR. Suppression of cystine uptake by sulfasalazine inhibits proliferation of human mammary carcinoma cells.
Anticancer Res 2003; 23: 4571-9.
PMID: 14981898
- Rappocciolo G, Hensler HR, Jais M, Reinhart TA, Pegu A, Jenkins FJ, Rinaldo CR. Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC-SIGN. J Virol 2008; 82: 4793-806.
PMID: 18337571
- Qiang W, Cahill JM, Liu J, Kuang X, Liu N, Scofield VL, Voorhees JR, Reid AJ, Yan M, Lynn WS, Wong PK. Activation of transcription factor Nrf-2 and its downstream targets in response to moloney murine leukemia virus ts1-induced thiol depletion and oxidative stress in astrocytes. J Virol 2004; 78: 11926-38.
PMID: 15479833
- Qiao HX, Hao CJ, Li Y, He X, Chen RS, Cui J, Xu ZH, Li W. JNK activation mediates the apoptosis of xCT-deficient cells. Biochem Biophys Res Commun 2008; 370: 584-8. PMID: 18395005
- Qin Z, Freitas E, Sullivan R, Mohan S, Bacelieri R, Branch D, Romano M, Kearney P, Oates J, Plaisance K, Renne R, Kaleeba J, Parsons C. Upregulation of xCT by KSHV-encoded microRNAs facilitates KSHV dissemination and persistence in an environment of oxidative stress. PLoS Pathog 2010; 6: e1000742. PMID: 20126446
- Sakakura Y, Sato H, Shiiya A, Tamba M, Sagara J, Matsuda M, Okamura N, Makino N, Bannai S.Expression and function of cystine/glutamate transporter in neutrophils. J Leukoc Biol 2007; 81: 974-82. PMID: 17200146
- Sasaki H, Sato H, Kuriyama-Matsumura K, Sato K, Maebara K, Wang H, Tamba M, Itoh K, Yamamoto M, Bannai S. Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J Biol Chem 2002; 277: 44765-71. PMID: 12235164
- Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins.
J Biol Chem 1999; 274:11455-8. PMID: 10206947
- Sato H, Tamba M, Kuriyama-Matsumura K, Okuno S, Bannai S. Molecular cloning and expression of human xCT, the light chain of amino acid transport system xc-.
Antioxid Redox Signal. 2000; 2:665-71. PMID: 11213471
- Sato H, Tamba M, Okuno S, Sato K, Keino-Masu K, Masu M, Bannai S. Distribution of cystine/glutamate exchange transporter, system x(c)-, in the mouse brain.
J Neurosci 2002; 22: 8028-33.PMID: 12223556
- Sato H, Nomura S, Maebara K, Sato K, Tamba M, Bannai S. Transcriptional control of cystine/glutamate transporter gene by amino acid deprivation.
Biochem Biophys Res Commun 2004; 325: 109-16. PMID: 155522208
- Savaskan NE, Heckel A, Hahnen E, Engelhorn T, Doerfler A, Ganslandt O, Nimsky C, Buchfelder M, Ey¨ąpoglu IY. Small interfering RNA-mediated xCT silencing in gliomas inhibits neurodegeneration and alleviates brain edema. Nat Med 2008;14: 629-32.PMID: 18469825
- Veettil MV, Sharma-Walia N, Sadagopan S, Raghu H, Sivakumar R, Naranatt PP, Chandran B. RhoA-GTPase facilitates entry of Kaposi's sarcoma-associated herpesvirus into adherent target cells in a Src-dependent manner.
J Virol 2006; 80: 11432-46. PMID: 17005646
- Veettil MV, Sadagopan S, Sharma-Walia N, Wang FZ, Raghu H, Varga L, Chandran B. Kaposi's sarcoma-associated herpesvirus forms a multimolecular complex of integrins (alphaVbeta5, alphaVbeta3, and alpha3beta1) and CD98-xCT during infection of human dermal microvascular endothelial cells, and CD98-xCT is essential for the postentry stage of infection. J Virol 2008; 82: 12126-44. PMID: 18829766
- Wang H, Tamba M, Kimata M, Sakamoto K, Bannai S, Sato H. Expression of the activity of cystine/glutamate exchange transporter, system x(c)(-), by xCT and rBAT.
Biochem Biophys Res Commun 2003; 305: 611-8. PMID: 12763038
- Zeng Y, Li Y, Chen RS, He X, Yang L, Li W. Overexpression of xCT induces up-regulation of 14-3-3beta in Kaposi's sarcoma. Biosci Rep 2010; 30: 277-83. PMID: 20100173
EDIT HISTORY:
Created by Wei Li, 08/18/2005
Updated by Wei Li, 04/06/2006
Updated by Wei Li, 12/28/2006
Updated by Wei Li, 08/20/2008
Updated by Wei Li, 03/12/2009
Updated by Wei Li, 05/29/2011
(assembly 05/2004)
SLC7A11: 12 exons, 71,737bp , chr4:139,449,092-139,520,828.
The figure below shows the structure of the SLC7A11 gene (data from UCSC genome browser).

Regulatory Element
Search the 5'UTR and 1kb upstream regions (seq1=human SLC7A11, seq2=mouse Slc7a11) by CONREAL with 80% Position Weight Matrices (PWMs) threshold (view results here). Two amino acid response elements (AAREs), each located in the opposite direction with an intervening sequence, were found in the 5'-flanking region of the mouse xCT gene (Sato, et al (2004)). The 5'-deletion analysis revealed that the sequence between -116 and -82 is essential for the basal expression and the sequence between -226 and -116 containing EpRE-1 is essential in response to diethyl maleate(Sasaki, et al).
TRANSCRIPT
RefSeq/ORF
SLC7A11(NM_014331), 3,144bp, view ORF and the alignment to genomic.
Expression Pattern
Tissue specificity: Widely expressed. It is expressed in Muller and retinal pigment epithelial cells, and a retinal ganglion cell line (RGC-5). Oxidative stress upregulates this transport system in RGC-5 cells (Dun, et al). Northern blot analysis demonstrated that the expression of both xCT and 4F2hc was significantly enhanced by oxygen. (Sato, et al (2000)).

BMR: Bone marrow; SPL: Spleen; TMS: Thymus; BRN: Brain; SPC: Spinal cord; HRT: Heart; MSL: Skeletal muscle; LVR; Liver; PNC: Pancreas; PST: Prostate; KDN: Kidney; LNG: Lung. (data from GeneCards )
PROTEIN
Sequence
xCT (NP_055146): 501aa, UniProtKB/Swiss-Prot entry Q9UPY5.
Synonym: Amino acid transport system xc-, Calcium channel blocker resistance protein CCBR1, Cystine/glutamate transporter, solute carrier family 7 (cationic amino acid transporter, y+ system) member 11
Ortholog
Species
Mouse Rat Dog Fowl GeneView
Slc7a11
Slc7a11
LOC483821
LOC428731
Protein
NP_036120 (502aa)
XP_227120 (502aa)
XP_540941 (551aa)
XP_426289 (580aa)
Identities
89% /500aa 87% /500aa 92% /482aa 68% /578aa
View multiple sequence alignment (PDF file) by ClustalW and GeneDoc.
Domain
(1) Domains predicted by SMART:
AA_permease: 48- 479
(2) Transmembrane domains predicted by SOSUI:
This amino acid sequence is of a MEMBRANE PROTEIN which have 13 transmembrane helices.
No. N terminal transmembrane region C terminal type length
1 46 LLRGVSIIIGTIIGAGIFISPKG 68 PRIMARY 23
2 75 SVGMSLTIWTVCGVLSLFGALSY 97 PRIMARY 23
3 109 GHYTYILEVFGPLPAFVRVWVEL 131 SECONDARY 23
4 136 PAATAVISLAFGRYILEPFFIQC 158 SECONDARY 23
5 162 ELAIKLITAVGITVVMVLNSMSV 184 PRIMARY 23
6 192 IFLTFCKLTAILIIIVPGVMQLI 214 PRIMARY 23
7 235 LPLAFYYGMYAYAGWFYLNFVTE 257 SECONDARY 23
8 268 LAICISMAIVTIGYVLTNVAYFT 290 PRIMARY 23
9 298 LLSNAVAVTFSERLLGNFSLAVP 320 SECONDARY 23
10 363 HTPLPAVIVLHPLTMIMLFSGDL 385 SECONDARY 23
11 389 LNFLSFARWLFIGLAVAGLIYLR 411 PRIMARY 23
12 422 KVPLFIPALFSFTCLFMVALSLY 444 PRIMARY 23
13 450 TGIGFVITLTGVPAYYLFIIWD 471 SECONDARY 22
Note: a topological model for xCT of 12 transmembrane domains with the N and C termini located inside the cell was proposed by Gasel et al .
(3) Graphic view of InterPro domain structure.
Motif/Site
(1) Predicted results by ScanProsite:
a) N-glycosylation site: 314 to 317 NFSL.
b) cAMP- and cGMP-dependent protein kinase phosphorylation site: 42 to 45 RKVT; 361 to 364 RKHT.
c) Protein kinase C phosphorylation site: 65 to 67 SPK; 103 to 105 TIK; 187 to 189 SAR; 226 to 228 SGR; 308 to 310 SER; 481 to 483 SEK.
d) Casein kinase II phosphorylation site: 96 to 99 SYAE; 226 to 229 SGRD; 306 to 309 TFSE.
e) N-myristoylation site: 9 sites.
(2) Predicted results of subprograms by PSORT II:
a) N-terminal signal peptide: none
b) 11 tentative TMs, membrane topology: type 3a
c) KDEL ER retention motif in C-terminus: none
d) ER membrane retention signals:
XXRR-like motif in the N-terminus: VRKP; KKXX-like motif in the C-terminus: EEDK
e) VAC possible vacuolar targeting motif: none
f) Actinin-type actin-binding motif: type 1: none; type 2: none
g) Prenylation motif: none
h) memYQRL transport motif from cell surface to Golgi: none
i) Tyrosines in the tail: none
j) Dileucine motif in the tail: none
3D Model
(1)ModBase predicted comparative 3D structure of Q9UPY5 from UCSC Genome Sorter.



From left to right: Front, Top, and Side views of predicted protein.
2D-PAGE
This protein does not exist in the current release of SWISS-2DPAGE.
Computed theoretical MW=55,423Da, pI=9.29 (NP_055146).
FUNCTION
Ontology
(1) Biological process: amino acid transport.
(2) Pheomelanin production.
(3) Oxidative stress.
(4) Belongs to the amino acid-polyamine-organocation (APC) superfamily. L-type amino acid transporter (LAT) (TC 2.A.3.8) family.
Location
Integral membrane protein. Transport system xc- was found in plasma membrane of cultured mammalian cells (Sato, et al (1999)).
Interaction
SLC7A11 (xCT), together with SLC3A2 (4F2hc, or CD98) linked by a disulfide bond at residue C158, encodes the heterodimeric amino acid transport system x(c)(-), which mediates cystine-glutamate exchange and thereby regulates intracellular glutathione levels. System x(c)- activity is important to maintain both intracellular glutathione levels and the redox balance between cystine and cysteine in the extracellular milieu. rBAT can replace 4F2hc in the expression of the activity of system x(c)(-) (Wang, et al).
In mouse NIH3T3 cells, the activity of system x(c)(-) and xCT mRNA is induced not only by deprivation of cystine but also by deprivation of other amino acids via a genomic cis-element termed amino acid response element (AARE) (Sato, et al (2004)). Overexpression of Nrf-2 in astrocytes specifically increases expression of the xCT cystine/glutamate antiporter and other thiol synthesis-related proteins, to promote cell survival (Qiang, et al). Cys(327) is a functionally important residue accessible to the aqueous extracellular environment and is structurally linked to the permeation pathway and/or the substrate binding site (Jimenez-Vidal, et al).
A CD44 variant (CD44v) interacts with xCT and controls the intracellular level of reduced glutathione (GSH). Ablation of CD44 induced loss of xCT from the cell surface and suppressed tumor growth in a transgenic mouse model of gastric cancer. It also induced activation of p38(MAPK), a downstream target of ROS, and expression of the gene for the cell cycle inhibitor p21(CIP1/WAF1)(Ishimoto, et al). xCT has been identified as a receptor mediating KSHV cell fusion (Kaleeba, et al).
miR-26b, which is down-regulated in human breast cancer specimens and cell lines, impairs viability and triggers apoptosis of human breast cancer MCF7 cells. SLC7A11 is identified as a direct target of miR-26b and its expression is remarkably increased in both breast cancer cell lines and clinical samples (Liu, et al).
Pathway
System x(c)(-) is a sodium-independent, high-affinity transporter of anionic amino acids with high specificity for anionic form of cystine and glutamate. Chintala, et al have reported that Slc7a11 regulates the pheomelanin production and cell proliferation. In melanosomes, dopaquinone reacts with cysteine to yield cysteinldopa, which is a precursor of pheomelanin. Cysteine is a component of the tripeptide glutathione. Glutathione metabolism pathway is described in KEGG Pathways.
MUTATION
Allele or SNP
No mutation has been reported in 32 human HPS patients who lack mutations in the seven known HPS genes (Chintala, et al).
SNPs deposited in dbSNP.
Distribution
(none)
Effect
(none)
PHENOTYPE
SLC7A11 is described in OMIM 607933.
Cystine uptake in human cancer cells is largely mediated by system Xc. xCT is relatively highly expressed in lung, colon, and central nervous system cancer cells (Huang, et al). Modulation of xCT activity is possiblely untilized in cancer therapy. Pharmacologic inhibition of system Xc remarkably inhibits the proliferation of several types of cancer cells, including lymphoma, glioma, prostate and breast cancer (Chung, et al; Doxsee, et al; Gout, et al; Narang, et al). This is thought be mediated by the depletion of GSH, which results in an increase of reactive oxygen species (ROS), and ultimately leads to caspase-dependent apoptosis (Doxsee, et al; Sakakura, et al). The apoptosis is likely mediated by c-Jun N-terminal kinase (JNK) activation. The JNK activation triggers both a caspase-dependent (caspases-9 and -3) and an ER stress-mediated (eIF2 and CHOP) pathway to induce apoptosis (Qiao, et al). In the absence of phospho-eIF2alpha, xCT expression is down-regulated that is mediated by nuclear factor ATF4, implicating the role eIF2alpha-ATF4-xCT module in neurodegeneration (Lewerenz, et al).
Disruption of xCT enhanced homotypic cell-cell adhesion and attenuated cell-extracellular matrix adhesion in an esophageal cancer cell line, KYSE150. SASP significantly inhibited both cell invasion of KYSE150 in vitro and its experimental metastasis in nude mice. Caveolin-1 was upregulated and beta-catenin was recruited to the plasma membrane when xCT was deficient, which were followed by the inhibition of beta-catenin transcriptional activity. Upregulation of caveolin-1 and inhibition of tumor cell invasion were mediated by reactive oxygen species-induced p38 MAPK activation (Chen, et al). Savaskan et al show that genetic or pharmacological inhibition of the glutamate transporter Xc system in gliomas leads to abrogated neurodegeneration, attenuated perifocal edema and prolonged survival. xCT deficiency causes unremitting inflammation because of the impaired survival of activated macrophages at the inflammatory site (Nabeyama, et al).
Kaposi's sarcoma-associated herpesvirus (KSHV, human herpesvirus 8) is the causative agent of Kaposi's sarcoma and other lymphoproliferative syndromes often associated with HIV/AIDS. xCT mediates the entry of KSHV which implicates its involvement in the infectious process of this pathogen (Kaleeba, et al; Veettil, et al (2006); Rappocciolo, et al). KSHV interacts with functionally related integrins (alphaVbeta3, alpha3beta1, and alphaVbeta5) and CD98/xCT molecules in a temporal fashion to form a multimolecular complex during the early stages of endothelial cell infection, probably mediating multiple roles in entry, signal transduction, and viral-gene expression (Veettil, et al (2008)). xCT was overexpressed in KS tissues and HHV-8-positive BCBL-1 cells. 14-3-3beta is a downstream effector of xCT in KS to mediate the cell proliferation (Zeng, et al). KSHV-encoded microRNAs (KSHV miRNAs) upregulate xCT expression by macrophages and endothelial cells, largely through miR-K12-11 suppression of BACH-1-a negative regulator of transcription recognizing antioxidant response elements within gene promoters (Qin, et al).
REFERENCE
- Chen RS, Song YM, Zhou ZY, Tong T, Li Y, Fu M, Guo XL, Dong LJ, He X, Qiao HX, Zhan QM, Li W. Disruption of xCT inhibits cancer cell metastasis via the caveolin-1/beta-catenin pathway. Oncogene 2009; 28: 599-609. PMID: 19015640
- Chintala S, Li W, Lamoreux ML, Ito S, Wakamatsu K, Sviderskaya EV, Bennett DC, Park YM, Gahl WA, Huizing M, Spritz RA, Ben S, Novak EK, Tan J, Swank RT.
Slc7a11 gene controls production of pheomelanin pigment and proliferation of cultured cells. Proc Natl Acad Sci U S A 2005; 102: 10964-9. PMID: 16037214
- Chung WJ, Lyons SA, Nelson GM, Hamza H, Gladson CL, Gillespie GY, Sontheimer H. Inhibition of cystine uptake disrupts the growth of primary brain tumors.
J Neurosci 2005; 25: 7101-10.
PMID: 16079392
- Doxsee DW, Gout PW, Kurita T, Lo M, Buckley AR, Wang Y, Xue H, Karp CM, Cutz JC, Cunha GR, Wang YZ. Sulfasalazine-induced cystine starvation: potential use for prostate cancer therapy. Prostate 2007; 67: 162-71.
PMID: 17075799
- Dun Y, Mysona B, Van Ells T, Amarnath L, Shamsul Ola M, Ganapathy V, Smith SB. Expression of the cystine-glutamate exchanger (x(c) (-)) in retinal ganglion cells and regulation by nitric oxide and oxidative stress. Cell Tissue Res 2006; 324: 189-202. PMID: 16444546
- Gasol E, Jimenez-Vidal M, Chillaron J, Zorzano A, Palacin M. Membrane topology of system xc- light subunit reveals a re-entrant loop with substrate-restricted accessibility.
J Biol Chem 2004; 279: 31228-36. PMID: 15151999
- Gout PW, Buckley AR, Simms CR, Bruchovsky N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the x(c)- cystine transporter: a new action for an old drug. Leukemia. 2001; 15: 1633-40.PMID: 11587223
- Huang Y, Dai Z, Barbacioru C, Sad¨¦e W. Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity and chemoresistance.Cancer Res 2005; 65: 7446-54.
PMID: 16103098
- Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, Masuko T, Shimizu T, Ishikawa T, Kai K, Takahashi E, Imamura Y, Baba Y, Ohmura M, Suematsu M, Baba H, Saya H. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 2011; 19: 387-400. PMID: 21397861
- Jimenez-Vidal M, Gasol E, Zorzano A, Nunes V, Palacin M, Chillaron J. Thiol modification of cysteine 327 in the eighth transmembrane domain of the light subunit xCT of the heteromeric cystine/glutamate antiporter suggests close proximity to the substrate binding site/permeation pathway.
J Biol Chem 2004; 279: 11214-21. PMID: 14722095
- Kaleeba JA, Berger EA. Kaposi's sarcoma-associated herpesvirus fusion-entry receptor: cystine transporter xCT. Science 2006; 311: 1921-4. PMID: 16574866
- Lewerenz J, Maher P. Basal levels of eIF2alpha phosphorylation determine cellular antioxidant status by regulating ATF4 and xCT expression. J Biol Chem 2009; 284: 1106-15. PMID: 19017641
- Liu XX, Li XJ, Zhang B, Liang YJ, Zhou CX, Cao DX, He M, Chen GQ, He JR, Zhao Q.
MicroRNA-26b is underexpressed in human breast cancer and induces cell apoptosis by targeting SLC7A11. FEBS Lett 2011; 585: 1363-7. PMID: 21510944
- Nabeyama A, Kurita A, Asano K, Miyake Y, Yasuda T, Miura I, Nishitai G, Arakawa S, Shimizu S, Wakana S, Yoshida H, Tanaka M. xCT deficiency accelerates chemically induced tumorigenesis. Proc Natl Acad Sci U S A 2010; 107: 6436-41.
PMID: 20308543
- Narang VS, Pauletti GM, Gout PW, Buckley DJ, Buckley AR. Suppression of cystine uptake by sulfasalazine inhibits proliferation of human mammary carcinoma cells.
Anticancer Res 2003; 23: 4571-9.
PMID: 14981898
- Rappocciolo G, Hensler HR, Jais M, Reinhart TA, Pegu A, Jenkins FJ, Rinaldo CR. Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC-SIGN. J Virol 2008; 82: 4793-806.
PMID: 18337571
- Qiang W, Cahill JM, Liu J, Kuang X, Liu N, Scofield VL, Voorhees JR, Reid AJ, Yan M, Lynn WS, Wong PK. Activation of transcription factor Nrf-2 and its downstream targets in response to moloney murine leukemia virus ts1-induced thiol depletion and oxidative stress in astrocytes. J Virol 2004; 78: 11926-38.
PMID: 15479833
- Qiao HX, Hao CJ, Li Y, He X, Chen RS, Cui J, Xu ZH, Li W. JNK activation mediates the apoptosis of xCT-deficient cells. Biochem Biophys Res Commun 2008; 370: 584-8. PMID: 18395005
- Qin Z, Freitas E, Sullivan R, Mohan S, Bacelieri R, Branch D, Romano M, Kearney P, Oates J, Plaisance K, Renne R, Kaleeba J, Parsons C. Upregulation of xCT by KSHV-encoded microRNAs facilitates KSHV dissemination and persistence in an environment of oxidative stress. PLoS Pathog 2010; 6: e1000742. PMID: 20126446
- Sakakura Y, Sato H, Shiiya A, Tamba M, Sagara J, Matsuda M, Okamura N, Makino N, Bannai S.Expression and function of cystine/glutamate transporter in neutrophils. J Leukoc Biol 2007; 81: 974-82. PMID: 17200146
- Sasaki H, Sato H, Kuriyama-Matsumura K, Sato K, Maebara K, Wang H, Tamba M, Itoh K, Yamamoto M, Bannai S. Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J Biol Chem 2002; 277: 44765-71. PMID: 12235164
- Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins.
J Biol Chem 1999; 274:11455-8. PMID: 10206947
- Sato H, Tamba M, Kuriyama-Matsumura K, Okuno S, Bannai S. Molecular cloning and expression of human xCT, the light chain of amino acid transport system xc-.
Antioxid Redox Signal. 2000; 2:665-71. PMID: 11213471
- Sato H, Tamba M, Okuno S, Sato K, Keino-Masu K, Masu M, Bannai S. Distribution of cystine/glutamate exchange transporter, system x(c)-, in the mouse brain.
J Neurosci 2002; 22: 8028-33.PMID: 12223556
- Sato H, Nomura S, Maebara K, Sato K, Tamba M, Bannai S. Transcriptional control of cystine/glutamate transporter gene by amino acid deprivation.
Biochem Biophys Res Commun 2004; 325: 109-16. PMID: 155522208
- Savaskan NE, Heckel A, Hahnen E, Engelhorn T, Doerfler A, Ganslandt O, Nimsky C, Buchfelder M, Ey¨ąpoglu IY. Small interfering RNA-mediated xCT silencing in gliomas inhibits neurodegeneration and alleviates brain edema. Nat Med 2008;14: 629-32.PMID: 18469825
- Veettil MV, Sharma-Walia N, Sadagopan S, Raghu H, Sivakumar R, Naranatt PP, Chandran B. RhoA-GTPase facilitates entry of Kaposi's sarcoma-associated herpesvirus into adherent target cells in a Src-dependent manner.
J Virol 2006; 80: 11432-46. PMID: 17005646
- Veettil MV, Sadagopan S, Sharma-Walia N, Wang FZ, Raghu H, Varga L, Chandran B. Kaposi's sarcoma-associated herpesvirus forms a multimolecular complex of integrins (alphaVbeta5, alphaVbeta3, and alpha3beta1) and CD98-xCT during infection of human dermal microvascular endothelial cells, and CD98-xCT is essential for the postentry stage of infection. J Virol 2008; 82: 12126-44. PMID: 18829766
- Wang H, Tamba M, Kimata M, Sakamoto K, Bannai S, Sato H. Expression of the activity of cystine/glutamate exchange transporter, system x(c)(-), by xCT and rBAT.
Biochem Biophys Res Commun 2003; 305: 611-8. PMID: 12763038
- Zeng Y, Li Y, Chen RS, He X, Yang L, Li W. Overexpression of xCT induces up-regulation of 14-3-3beta in Kaposi's sarcoma. Biosci Rep 2010; 30: 277-83. PMID: 20100173
EDIT HISTORY:
Created by Wei Li, 08/18/2005
Updated by Wei Li, 04/06/2006
Updated by Wei Li, 12/28/2006
Updated by Wei Li, 08/20/2008
Updated by Wei Li, 03/12/2009
Updated by Wei Li, 05/29/2011
Search the 5'UTR and 1kb upstream regions (seq1=human SLC7A11, seq2=mouse Slc7a11) by CONREAL with 80% Position Weight Matrices (PWMs) threshold (view results here). Two amino acid response elements (AAREs), each located in the opposite direction with an intervening sequence, were found in the 5'-flanking region of the mouse xCT gene (Sato, et al (2004)). The 5'-deletion analysis revealed that the sequence between -116 and -82 is essential for the basal expression and the sequence between -226 and -116 containing EpRE-1 is essential in response to diethyl maleate(Sasaki, et al).
TRANSCRIPT
RefSeq/ORF
SLC7A11(NM_014331), 3,144bp, view ORF and the alignment to genomic.
Expression Pattern
Tissue specificity: Widely expressed. It is expressed in Muller and retinal pigment epithelial cells, and a retinal ganglion cell line (RGC-5). Oxidative stress upregulates this transport system in RGC-5 cells (Dun, et al). Northern blot analysis demonstrated that the expression of both xCT and 4F2hc was significantly enhanced by oxygen. (Sato, et al (2000)).

BMR: Bone marrow; SPL: Spleen; TMS: Thymus; BRN: Brain; SPC: Spinal cord; HRT: Heart; MSL: Skeletal muscle; LVR; Liver; PNC: Pancreas; PST: Prostate; KDN: Kidney; LNG: Lung. (data from GeneCards )
PROTEIN
Sequence
xCT (NP_055146): 501aa, UniProtKB/Swiss-Prot entry Q9UPY5.
Synonym: Amino acid transport system xc-, Calcium channel blocker resistance protein CCBR1, Cystine/glutamate transporter, solute carrier family 7 (cationic amino acid transporter, y+ system) member 11
Ortholog
Species
Mouse Rat Dog Fowl GeneView
Slc7a11
Slc7a11
LOC483821
LOC428731
Protein
NP_036120 (502aa)
XP_227120 (502aa)
XP_540941 (551aa)
XP_426289 (580aa)
Identities
89% /500aa 87% /500aa 92% /482aa 68% /578aa
View multiple sequence alignment (PDF file) by ClustalW and GeneDoc.
Domain
(1) Domains predicted by SMART:
AA_permease: 48- 479
(2) Transmembrane domains predicted by SOSUI:
This amino acid sequence is of a MEMBRANE PROTEIN which have 13 transmembrane helices.
No. N terminal transmembrane region C terminal type length
1 46 LLRGVSIIIGTIIGAGIFISPKG 68 PRIMARY 23
2 75 SVGMSLTIWTVCGVLSLFGALSY 97 PRIMARY 23
3 109 GHYTYILEVFGPLPAFVRVWVEL 131 SECONDARY 23
4 136 PAATAVISLAFGRYILEPFFIQC 158 SECONDARY 23
5 162 ELAIKLITAVGITVVMVLNSMSV 184 PRIMARY 23
6 192 IFLTFCKLTAILIIIVPGVMQLI 214 PRIMARY 23
7 235 LPLAFYYGMYAYAGWFYLNFVTE 257 SECONDARY 23
8 268 LAICISMAIVTIGYVLTNVAYFT 290 PRIMARY 23
9 298 LLSNAVAVTFSERLLGNFSLAVP 320 SECONDARY 23
10 363 HTPLPAVIVLHPLTMIMLFSGDL 385 SECONDARY 23
11 389 LNFLSFARWLFIGLAVAGLIYLR 411 PRIMARY 23
12 422 KVPLFIPALFSFTCLFMVALSLY 444 PRIMARY 23
13 450 TGIGFVITLTGVPAYYLFIIWD 471 SECONDARY 22
Note: a topological model for xCT of 12 transmembrane domains with the N and C termini located inside the cell was proposed by Gasel et al .
(3) Graphic view of InterPro domain structure.
Motif/Site
(1) Predicted results by ScanProsite:
a) N-glycosylation site: 314 to 317 NFSL.
b) cAMP- and cGMP-dependent protein kinase phosphorylation site: 42 to 45 RKVT; 361 to 364 RKHT.
c) Protein kinase C phosphorylation site: 65 to 67 SPK; 103 to 105 TIK; 187 to 189 SAR; 226 to 228 SGR; 308 to 310 SER; 481 to 483 SEK.
d) Casein kinase II phosphorylation site: 96 to 99 SYAE; 226 to 229 SGRD; 306 to 309 TFSE.
e) N-myristoylation site: 9 sites.
(2) Predicted results of subprograms by PSORT II:
a) N-terminal signal peptide: none
b) 11 tentative TMs, membrane topology: type 3a
c) KDEL ER retention motif in C-terminus: none
d) ER membrane retention signals:
XXRR-like motif in the N-terminus: VRKP; KKXX-like motif in the C-terminus: EEDK
e) VAC possible vacuolar targeting motif: none
f) Actinin-type actin-binding motif: type 1: none; type 2: none
g) Prenylation motif: none
h) memYQRL transport motif from cell surface to Golgi: none
i) Tyrosines in the tail: none
j) Dileucine motif in the tail: none
3D Model
(1)ModBase predicted comparative 3D structure of Q9UPY5 from UCSC Genome Sorter.



From left to right: Front, Top, and Side views of predicted protein.
2D-PAGE
This protein does not exist in the current release of SWISS-2DPAGE.
Computed theoretical MW=55,423Da, pI=9.29 (NP_055146).
FUNCTION
Ontology
(1) Biological process: amino acid transport.
(2) Pheomelanin production.
(3) Oxidative stress.
(4) Belongs to the amino acid-polyamine-organocation (APC) superfamily. L-type amino acid transporter (LAT) (TC 2.A.3.8) family.
Location
Integral membrane protein. Transport system xc- was found in plasma membrane of cultured mammalian cells (Sato, et al (1999)).
Interaction
SLC7A11 (xCT), together with SLC3A2 (4F2hc, or CD98) linked by a disulfide bond at residue C158, encodes the heterodimeric amino acid transport system x(c)(-), which mediates cystine-glutamate exchange and thereby regulates intracellular glutathione levels. System x(c)- activity is important to maintain both intracellular glutathione levels and the redox balance between cystine and cysteine in the extracellular milieu. rBAT can replace 4F2hc in the expression of the activity of system x(c)(-) (Wang, et al).
In mouse NIH3T3 cells, the activity of system x(c)(-) and xCT mRNA is induced not only by deprivation of cystine but also by deprivation of other amino acids via a genomic cis-element termed amino acid response element (AARE) (Sato, et al (2004)). Overexpression of Nrf-2 in astrocytes specifically increases expression of the xCT cystine/glutamate antiporter and other thiol synthesis-related proteins, to promote cell survival (Qiang, et al). Cys(327) is a functionally important residue accessible to the aqueous extracellular environment and is structurally linked to the permeation pathway and/or the substrate binding site (Jimenez-Vidal, et al).
A CD44 variant (CD44v) interacts with xCT and controls the intracellular level of reduced glutathione (GSH). Ablation of CD44 induced loss of xCT from the cell surface and suppressed tumor growth in a transgenic mouse model of gastric cancer. It also induced activation of p38(MAPK), a downstream target of ROS, and expression of the gene for the cell cycle inhibitor p21(CIP1/WAF1)(Ishimoto, et al). xCT has been identified as a receptor mediating KSHV cell fusion (Kaleeba, et al).
miR-26b, which is down-regulated in human breast cancer specimens and cell lines, impairs viability and triggers apoptosis of human breast cancer MCF7 cells. SLC7A11 is identified as a direct target of miR-26b and its expression is remarkably increased in both breast cancer cell lines and clinical samples (Liu, et al).
Pathway
System x(c)(-) is a sodium-independent, high-affinity transporter of anionic amino acids with high specificity for anionic form of cystine and glutamate. Chintala, et al have reported that Slc7a11 regulates the pheomelanin production and cell proliferation. In melanosomes, dopaquinone reacts with cysteine to yield cysteinldopa, which is a precursor of pheomelanin. Cysteine is a component of the tripeptide glutathione. Glutathione metabolism pathway is described in KEGG Pathways.
MUTATION
Allele or SNP
No mutation has been reported in 32 human HPS patients who lack mutations in the seven known HPS genes (Chintala, et al).
SNPs deposited in dbSNP.
Distribution
(none)
Effect
(none)
PHENOTYPE
SLC7A11 is described in OMIM 607933.
Cystine uptake in human cancer cells is largely mediated by system Xc. xCT is relatively highly expressed in lung, colon, and central nervous system cancer cells (Huang, et al). Modulation of xCT activity is possiblely untilized in cancer therapy. Pharmacologic inhibition of system Xc remarkably inhibits the proliferation of several types of cancer cells, including lymphoma, glioma, prostate and breast cancer (Chung, et al; Doxsee, et al; Gout, et al; Narang, et al). This is thought be mediated by the depletion of GSH, which results in an increase of reactive oxygen species (ROS), and ultimately leads to caspase-dependent apoptosis (Doxsee, et al; Sakakura, et al). The apoptosis is likely mediated by c-Jun N-terminal kinase (JNK) activation. The JNK activation triggers both a caspase-dependent (caspases-9 and -3) and an ER stress-mediated (eIF2 and CHOP) pathway to induce apoptosis (Qiao, et al). In the absence of phospho-eIF2alpha, xCT expression is down-regulated that is mediated by nuclear factor ATF4, implicating the role eIF2alpha-ATF4-xCT module in neurodegeneration (Lewerenz, et al).
Disruption of xCT enhanced homotypic cell-cell adhesion and attenuated cell-extracellular matrix adhesion in an esophageal cancer cell line, KYSE150. SASP significantly inhibited both cell invasion of KYSE150 in vitro and its experimental metastasis in nude mice. Caveolin-1 was upregulated and beta-catenin was recruited to the plasma membrane when xCT was deficient, which were followed by the inhibition of beta-catenin transcriptional activity. Upregulation of caveolin-1 and inhibition of tumor cell invasion were mediated by reactive oxygen species-induced p38 MAPK activation (Chen, et al). Savaskan et al show that genetic or pharmacological inhibition of the glutamate transporter Xc system in gliomas leads to abrogated neurodegeneration, attenuated perifocal edema and prolonged survival. xCT deficiency causes unremitting inflammation because of the impaired survival of activated macrophages at the inflammatory site (Nabeyama, et al).
Kaposi's sarcoma-associated herpesvirus (KSHV, human herpesvirus 8) is the causative agent of Kaposi's sarcoma and other lymphoproliferative syndromes often associated with HIV/AIDS. xCT mediates the entry of KSHV which implicates its involvement in the infectious process of this pathogen (Kaleeba, et al; Veettil, et al (2006); Rappocciolo, et al). KSHV interacts with functionally related integrins (alphaVbeta3, alpha3beta1, and alphaVbeta5) and CD98/xCT molecules in a temporal fashion to form a multimolecular complex during the early stages of endothelial cell infection, probably mediating multiple roles in entry, signal transduction, and viral-gene expression (Veettil, et al (2008)). xCT was overexpressed in KS tissues and HHV-8-positive BCBL-1 cells. 14-3-3beta is a downstream effector of xCT in KS to mediate the cell proliferation (Zeng, et al). KSHV-encoded microRNAs (KSHV miRNAs) upregulate xCT expression by macrophages and endothelial cells, largely through miR-K12-11 suppression of BACH-1-a negative regulator of transcription recognizing antioxidant response elements within gene promoters (Qin, et al).
REFERENCE
- Chen RS, Song YM, Zhou ZY, Tong T, Li Y, Fu M, Guo XL, Dong LJ, He X, Qiao HX, Zhan QM, Li W. Disruption of xCT inhibits cancer cell metastasis via the caveolin-1/beta-catenin pathway. Oncogene 2009; 28: 599-609. PMID: 19015640
- Chintala S, Li W, Lamoreux ML, Ito S, Wakamatsu K, Sviderskaya EV, Bennett DC, Park YM, Gahl WA, Huizing M, Spritz RA, Ben S, Novak EK, Tan J, Swank RT.
Slc7a11 gene controls production of pheomelanin pigment and proliferation of cultured cells. Proc Natl Acad Sci U S A 2005; 102: 10964-9. PMID: 16037214
- Chung WJ, Lyons SA, Nelson GM, Hamza H, Gladson CL, Gillespie GY, Sontheimer H. Inhibition of cystine uptake disrupts the growth of primary brain tumors.
J Neurosci 2005; 25: 7101-10.
PMID: 16079392
- Doxsee DW, Gout PW, Kurita T, Lo M, Buckley AR, Wang Y, Xue H, Karp CM, Cutz JC, Cunha GR, Wang YZ. Sulfasalazine-induced cystine starvation: potential use for prostate cancer therapy. Prostate 2007; 67: 162-71.
PMID: 17075799
- Dun Y, Mysona B, Van Ells T, Amarnath L, Shamsul Ola M, Ganapathy V, Smith SB. Expression of the cystine-glutamate exchanger (x(c) (-)) in retinal ganglion cells and regulation by nitric oxide and oxidative stress. Cell Tissue Res 2006; 324: 189-202. PMID: 16444546
- Gasol E, Jimenez-Vidal M, Chillaron J, Zorzano A, Palacin M. Membrane topology of system xc- light subunit reveals a re-entrant loop with substrate-restricted accessibility.
J Biol Chem 2004; 279: 31228-36. PMID: 15151999
- Gout PW, Buckley AR, Simms CR, Bruchovsky N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the x(c)- cystine transporter: a new action for an old drug. Leukemia. 2001; 15: 1633-40.PMID: 11587223
- Huang Y, Dai Z, Barbacioru C, Sad¨¦e W. Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity and chemoresistance.Cancer Res 2005; 65: 7446-54.
PMID: 16103098
- Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, Masuko T, Shimizu T, Ishikawa T, Kai K, Takahashi E, Imamura Y, Baba Y, Ohmura M, Suematsu M, Baba H, Saya H. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 2011; 19: 387-400. PMID: 21397861
- Jimenez-Vidal M, Gasol E, Zorzano A, Nunes V, Palacin M, Chillaron J. Thiol modification of cysteine 327 in the eighth transmembrane domain of the light subunit xCT of the heteromeric cystine/glutamate antiporter suggests close proximity to the substrate binding site/permeation pathway.
J Biol Chem 2004; 279: 11214-21. PMID: 14722095
- Kaleeba JA, Berger EA. Kaposi's sarcoma-associated herpesvirus fusion-entry receptor: cystine transporter xCT. Science 2006; 311: 1921-4. PMID: 16574866
- Lewerenz J, Maher P. Basal levels of eIF2alpha phosphorylation determine cellular antioxidant status by regulating ATF4 and xCT expression. J Biol Chem 2009; 284: 1106-15. PMID: 19017641
- Liu XX, Li XJ, Zhang B, Liang YJ, Zhou CX, Cao DX, He M, Chen GQ, He JR, Zhao Q.
MicroRNA-26b is underexpressed in human breast cancer and induces cell apoptosis by targeting SLC7A11. FEBS Lett 2011; 585: 1363-7. PMID: 21510944
- Nabeyama A, Kurita A, Asano K, Miyake Y, Yasuda T, Miura I, Nishitai G, Arakawa S, Shimizu S, Wakana S, Yoshida H, Tanaka M. xCT deficiency accelerates chemically induced tumorigenesis. Proc Natl Acad Sci U S A 2010; 107: 6436-41.
PMID: 20308543
- Narang VS, Pauletti GM, Gout PW, Buckley DJ, Buckley AR. Suppression of cystine uptake by sulfasalazine inhibits proliferation of human mammary carcinoma cells.
Anticancer Res 2003; 23: 4571-9.
PMID: 14981898
- Rappocciolo G, Hensler HR, Jais M, Reinhart TA, Pegu A, Jenkins FJ, Rinaldo CR. Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC-SIGN. J Virol 2008; 82: 4793-806.
PMID: 18337571
- Qiang W, Cahill JM, Liu J, Kuang X, Liu N, Scofield VL, Voorhees JR, Reid AJ, Yan M, Lynn WS, Wong PK. Activation of transcription factor Nrf-2 and its downstream targets in response to moloney murine leukemia virus ts1-induced thiol depletion and oxidative stress in astrocytes. J Virol 2004; 78: 11926-38.
PMID: 15479833
- Qiao HX, Hao CJ, Li Y, He X, Chen RS, Cui J, Xu ZH, Li W. JNK activation mediates the apoptosis of xCT-deficient cells. Biochem Biophys Res Commun 2008; 370: 584-8. PMID: 18395005
- Qin Z, Freitas E, Sullivan R, Mohan S, Bacelieri R, Branch D, Romano M, Kearney P, Oates J, Plaisance K, Renne R, Kaleeba J, Parsons C. Upregulation of xCT by KSHV-encoded microRNAs facilitates KSHV dissemination and persistence in an environment of oxidative stress. PLoS Pathog 2010; 6: e1000742. PMID: 20126446
- Sakakura Y, Sato H, Shiiya A, Tamba M, Sagara J, Matsuda M, Okamura N, Makino N, Bannai S.Expression and function of cystine/glutamate transporter in neutrophils. J Leukoc Biol 2007; 81: 974-82. PMID: 17200146
- Sasaki H, Sato H, Kuriyama-Matsumura K, Sato K, Maebara K, Wang H, Tamba M, Itoh K, Yamamoto M, Bannai S. Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J Biol Chem 2002; 277: 44765-71. PMID: 12235164
- Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins.
J Biol Chem 1999; 274:11455-8. PMID: 10206947
- Sato H, Tamba M, Kuriyama-Matsumura K, Okuno S, Bannai S. Molecular cloning and expression of human xCT, the light chain of amino acid transport system xc-.
Antioxid Redox Signal. 2000; 2:665-71. PMID: 11213471
- Sato H, Tamba M, Okuno S, Sato K, Keino-Masu K, Masu M, Bannai S. Distribution of cystine/glutamate exchange transporter, system x(c)-, in the mouse brain.
J Neurosci 2002; 22: 8028-33.PMID: 12223556
- Sato H, Nomura S, Maebara K, Sato K, Tamba M, Bannai S. Transcriptional control of cystine/glutamate transporter gene by amino acid deprivation.
Biochem Biophys Res Commun 2004; 325: 109-16. PMID: 155522208
- Savaskan NE, Heckel A, Hahnen E, Engelhorn T, Doerfler A, Ganslandt O, Nimsky C, Buchfelder M, Ey¨ąpoglu IY. Small interfering RNA-mediated xCT silencing in gliomas inhibits neurodegeneration and alleviates brain edema. Nat Med 2008;14: 629-32.PMID: 18469825
- Veettil MV, Sharma-Walia N, Sadagopan S, Raghu H, Sivakumar R, Naranatt PP, Chandran B. RhoA-GTPase facilitates entry of Kaposi's sarcoma-associated herpesvirus into adherent target cells in a Src-dependent manner.
J Virol 2006; 80: 11432-46. PMID: 17005646
- Veettil MV, Sadagopan S, Sharma-Walia N, Wang FZ, Raghu H, Varga L, Chandran B. Kaposi's sarcoma-associated herpesvirus forms a multimolecular complex of integrins (alphaVbeta5, alphaVbeta3, and alpha3beta1) and CD98-xCT during infection of human dermal microvascular endothelial cells, and CD98-xCT is essential for the postentry stage of infection. J Virol 2008; 82: 12126-44. PMID: 18829766
- Wang H, Tamba M, Kimata M, Sakamoto K, Bannai S, Sato H. Expression of the activity of cystine/glutamate exchange transporter, system x(c)(-), by xCT and rBAT.
Biochem Biophys Res Commun 2003; 305: 611-8. PMID: 12763038
- Zeng Y, Li Y, Chen RS, He X, Yang L, Li W. Overexpression of xCT induces up-regulation of 14-3-3beta in Kaposi's sarcoma. Biosci Rep 2010; 30: 277-83. PMID: 20100173
EDIT HISTORY:
Created by Wei Li, 08/18/2005
Updated by Wei Li, 04/06/2006
Updated by Wei Li, 12/28/2006
Updated by Wei Li, 08/20/2008
Updated by Wei Li, 03/12/2009
Updated by Wei Li, 05/29/2011
SLC7A11(NM_014331), 3,144bp, view ORF and the alignment to genomic.
Expression Pattern
Tissue specificity: Widely expressed. It is expressed in Muller and retinal pigment epithelial cells, and a retinal ganglion cell line (RGC-5). Oxidative stress upregulates this transport system in RGC-5 cells (Dun, et al). Northern blot analysis demonstrated that the expression of both xCT and 4F2hc was significantly enhanced by oxygen. (Sato, et al (2000)).

BMR: Bone marrow; SPL: Spleen; TMS: Thymus; BRN: Brain; SPC: Spinal cord; HRT: Heart; MSL: Skeletal muscle; LVR; Liver; PNC: Pancreas; PST: Prostate; KDN: Kidney; LNG: Lung. (data from GeneCards )
PROTEIN
Sequence
xCT (NP_055146): 501aa, UniProtKB/Swiss-Prot entry Q9UPY5.
Synonym: Amino acid transport system xc-, Calcium channel blocker resistance protein CCBR1, Cystine/glutamate transporter, solute carrier family 7 (cationic amino acid transporter, y+ system) member 11
Ortholog
Species
Mouse Rat Dog Fowl GeneView
Slc7a11
Slc7a11
LOC483821
LOC428731
Protein
NP_036120 (502aa)
XP_227120 (502aa)
XP_540941 (551aa)
XP_426289 (580aa)
Identities
89% /500aa 87% /500aa 92% /482aa 68% /578aa
View multiple sequence alignment (PDF file) by ClustalW and GeneDoc.
Domain
(1) Domains predicted by SMART:
AA_permease: 48- 479
(2) Transmembrane domains predicted by SOSUI:
This amino acid sequence is of a MEMBRANE PROTEIN which have 13 transmembrane helices.
No. N terminal transmembrane region C terminal type length
1 46 LLRGVSIIIGTIIGAGIFISPKG 68 PRIMARY 23
2 75 SVGMSLTIWTVCGVLSLFGALSY 97 PRIMARY 23
3 109 GHYTYILEVFGPLPAFVRVWVEL 131 SECONDARY 23
4 136 PAATAVISLAFGRYILEPFFIQC 158 SECONDARY 23
5 162 ELAIKLITAVGITVVMVLNSMSV 184 PRIMARY 23
6 192 IFLTFCKLTAILIIIVPGVMQLI 214 PRIMARY 23
7 235 LPLAFYYGMYAYAGWFYLNFVTE 257 SECONDARY 23
8 268 LAICISMAIVTIGYVLTNVAYFT 290 PRIMARY 23
9 298 LLSNAVAVTFSERLLGNFSLAVP 320 SECONDARY 23
10 363 HTPLPAVIVLHPLTMIMLFSGDL 385 SECONDARY 23
11 389 LNFLSFARWLFIGLAVAGLIYLR 411 PRIMARY 23
12 422 KVPLFIPALFSFTCLFMVALSLY 444 PRIMARY 23
13 450 TGIGFVITLTGVPAYYLFIIWD 471 SECONDARY 22
Note: a topological model for xCT of 12 transmembrane domains with the N and C termini located inside the cell was proposed by Gasel et al .
(3) Graphic view of InterPro domain structure.
Motif/Site
(1) Predicted results by ScanProsite:
a) N-glycosylation site: 314 to 317 NFSL.
b) cAMP- and cGMP-dependent protein kinase phosphorylation site: 42 to 45 RKVT; 361 to 364 RKHT.
c) Protein kinase C phosphorylation site: 65 to 67 SPK; 103 to 105 TIK; 187 to 189 SAR; 226 to 228 SGR; 308 to 310 SER; 481 to 483 SEK.
d) Casein kinase II phosphorylation site: 96 to 99 SYAE; 226 to 229 SGRD; 306 to 309 TFSE.
e) N-myristoylation site: 9 sites.
(2) Predicted results of subprograms by PSORT II:
a) N-terminal signal peptide: none
b) 11 tentative TMs, membrane topology: type 3a
c) KDEL ER retention motif in C-terminus: none
d) ER membrane retention signals:
XXRR-like motif in the N-terminus: VRKP; KKXX-like motif in the C-terminus: EEDK
e) VAC possible vacuolar targeting motif: none
f) Actinin-type actin-binding motif: type 1: none; type 2: none
g) Prenylation motif: none
h) memYQRL transport motif from cell surface to Golgi: none
i) Tyrosines in the tail: none
j) Dileucine motif in the tail: none
3D Model
(1)ModBase predicted comparative 3D structure of Q9UPY5 from UCSC Genome Sorter.



From left to right: Front, Top, and Side views of predicted protein.
2D-PAGE
This protein does not exist in the current release of SWISS-2DPAGE.
Computed theoretical MW=55,423Da, pI=9.29 (NP_055146).
FUNCTION
Ontology
(1) Biological process: amino acid transport.
(2) Pheomelanin production.
(3) Oxidative stress.
(4) Belongs to the amino acid-polyamine-organocation (APC) superfamily. L-type amino acid transporter (LAT) (TC 2.A.3.8) family.
Location
Integral membrane protein. Transport system xc- was found in plasma membrane of cultured mammalian cells (Sato, et al (1999)).
Interaction
SLC7A11 (xCT), together with SLC3A2 (4F2hc, or CD98) linked by a disulfide bond at residue C158, encodes the heterodimeric amino acid transport system x(c)(-), which mediates cystine-glutamate exchange and thereby regulates intracellular glutathione levels. System x(c)- activity is important to maintain both intracellular glutathione levels and the redox balance between cystine and cysteine in the extracellular milieu. rBAT can replace 4F2hc in the expression of the activity of system x(c)(-) (Wang, et al).
In mouse NIH3T3 cells, the activity of system x(c)(-) and xCT mRNA is induced not only by deprivation of cystine but also by deprivation of other amino acids via a genomic cis-element termed amino acid response element (AARE) (Sato, et al (2004)). Overexpression of Nrf-2 in astrocytes specifically increases expression of the xCT cystine/glutamate antiporter and other thiol synthesis-related proteins, to promote cell survival (Qiang, et al). Cys(327) is a functionally important residue accessible to the aqueous extracellular environment and is structurally linked to the permeation pathway and/or the substrate binding site (Jimenez-Vidal, et al).
A CD44 variant (CD44v) interacts with xCT and controls the intracellular level of reduced glutathione (GSH). Ablation of CD44 induced loss of xCT from the cell surface and suppressed tumor growth in a transgenic mouse model of gastric cancer. It also induced activation of p38(MAPK), a downstream target of ROS, and expression of the gene for the cell cycle inhibitor p21(CIP1/WAF1)(Ishimoto, et al). xCT has been identified as a receptor mediating KSHV cell fusion (Kaleeba, et al).
miR-26b, which is down-regulated in human breast cancer specimens and cell lines, impairs viability and triggers apoptosis of human breast cancer MCF7 cells. SLC7A11 is identified as a direct target of miR-26b and its expression is remarkably increased in both breast cancer cell lines and clinical samples (Liu, et al).
Pathway
System x(c)(-) is a sodium-independent, high-affinity transporter of anionic amino acids with high specificity for anionic form of cystine and glutamate. Chintala, et al have reported that Slc7a11 regulates the pheomelanin production and cell proliferation. In melanosomes, dopaquinone reacts with cysteine to yield cysteinldopa, which is a precursor of pheomelanin. Cysteine is a component of the tripeptide glutathione. Glutathione metabolism pathway is described in KEGG Pathways.
MUTATION
Allele or SNP
No mutation has been reported in 32 human HPS patients who lack mutations in the seven known HPS genes (Chintala, et al).
SNPs deposited in dbSNP.
Distribution
(none)
Effect
(none)
PHENOTYPE
SLC7A11 is described in OMIM 607933.
Cystine uptake in human cancer cells is largely mediated by system Xc. xCT is relatively highly expressed in lung, colon, and central nervous system cancer cells (Huang, et al). Modulation of xCT activity is possiblely untilized in cancer therapy. Pharmacologic inhibition of system Xc remarkably inhibits the proliferation of several types of cancer cells, including lymphoma, glioma, prostate and breast cancer (Chung, et al; Doxsee, et al; Gout, et al; Narang, et al). This is thought be mediated by the depletion of GSH, which results in an increase of reactive oxygen species (ROS), and ultimately leads to caspase-dependent apoptosis (Doxsee, et al; Sakakura, et al). The apoptosis is likely mediated by c-Jun N-terminal kinase (JNK) activation. The JNK activation triggers both a caspase-dependent (caspases-9 and -3) and an ER stress-mediated (eIF2 and CHOP) pathway to induce apoptosis (Qiao, et al). In the absence of phospho-eIF2alpha, xCT expression is down-regulated that is mediated by nuclear factor ATF4, implicating the role eIF2alpha-ATF4-xCT module in neurodegeneration (Lewerenz, et al).
Disruption of xCT enhanced homotypic cell-cell adhesion and attenuated cell-extracellular matrix adhesion in an esophageal cancer cell line, KYSE150. SASP significantly inhibited both cell invasion of KYSE150 in vitro and its experimental metastasis in nude mice. Caveolin-1 was upregulated and beta-catenin was recruited to the plasma membrane when xCT was deficient, which were followed by the inhibition of beta-catenin transcriptional activity. Upregulation of caveolin-1 and inhibition of tumor cell invasion were mediated by reactive oxygen species-induced p38 MAPK activation (Chen, et al). Savaskan et al show that genetic or pharmacological inhibition of the glutamate transporter Xc system in gliomas leads to abrogated neurodegeneration, attenuated perifocal edema and prolonged survival. xCT deficiency causes unremitting inflammation because of the impaired survival of activated macrophages at the inflammatory site (Nabeyama, et al).
Kaposi's sarcoma-associated herpesvirus (KSHV, human herpesvirus 8) is the causative agent of Kaposi's sarcoma and other lymphoproliferative syndromes often associated with HIV/AIDS. xCT mediates the entry of KSHV which implicates its involvement in the infectious process of this pathogen (Kaleeba, et al; Veettil, et al (2006); Rappocciolo, et al). KSHV interacts with functionally related integrins (alphaVbeta3, alpha3beta1, and alphaVbeta5) and CD98/xCT molecules in a temporal fashion to form a multimolecular complex during the early stages of endothelial cell infection, probably mediating multiple roles in entry, signal transduction, and viral-gene expression (Veettil, et al (2008)). xCT was overexpressed in KS tissues and HHV-8-positive BCBL-1 cells. 14-3-3beta is a downstream effector of xCT in KS to mediate the cell proliferation (Zeng, et al). KSHV-encoded microRNAs (KSHV miRNAs) upregulate xCT expression by macrophages and endothelial cells, largely through miR-K12-11 suppression of BACH-1-a negative regulator of transcription recognizing antioxidant response elements within gene promoters (Qin, et al).
REFERENCE
- Chen RS, Song YM, Zhou ZY, Tong T, Li Y, Fu M, Guo XL, Dong LJ, He X, Qiao HX, Zhan QM, Li W. Disruption of xCT inhibits cancer cell metastasis via the caveolin-1/beta-catenin pathway. Oncogene 2009; 28: 599-609. PMID: 19015640
- Chintala S, Li W, Lamoreux ML, Ito S, Wakamatsu K, Sviderskaya EV, Bennett DC, Park YM, Gahl WA, Huizing M, Spritz RA, Ben S, Novak EK, Tan J, Swank RT.
Slc7a11 gene controls production of pheomelanin pigment and proliferation of cultured cells. Proc Natl Acad Sci U S A 2005; 102: 10964-9. PMID: 16037214
- Chung WJ, Lyons SA, Nelson GM, Hamza H, Gladson CL, Gillespie GY, Sontheimer H. Inhibition of cystine uptake disrupts the growth of primary brain tumors.
J Neurosci 2005; 25: 7101-10.
PMID: 16079392
- Doxsee DW, Gout PW, Kurita T, Lo M, Buckley AR, Wang Y, Xue H, Karp CM, Cutz JC, Cunha GR, Wang YZ. Sulfasalazine-induced cystine starvation: potential use for prostate cancer therapy. Prostate 2007; 67: 162-71.
PMID: 17075799
- Dun Y, Mysona B, Van Ells T, Amarnath L, Shamsul Ola M, Ganapathy V, Smith SB. Expression of the cystine-glutamate exchanger (x(c) (-)) in retinal ganglion cells and regulation by nitric oxide and oxidative stress. Cell Tissue Res 2006; 324: 189-202. PMID: 16444546
- Gasol E, Jimenez-Vidal M, Chillaron J, Zorzano A, Palacin M. Membrane topology of system xc- light subunit reveals a re-entrant loop with substrate-restricted accessibility.
J Biol Chem 2004; 279: 31228-36. PMID: 15151999
- Gout PW, Buckley AR, Simms CR, Bruchovsky N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the x(c)- cystine transporter: a new action for an old drug. Leukemia. 2001; 15: 1633-40.PMID: 11587223
- Huang Y, Dai Z, Barbacioru C, Sad¨¦e W. Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity and chemoresistance.Cancer Res 2005; 65: 7446-54.
PMID: 16103098
- Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, Masuko T, Shimizu T, Ishikawa T, Kai K, Takahashi E, Imamura Y, Baba Y, Ohmura M, Suematsu M, Baba H, Saya H. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 2011; 19: 387-400. PMID: 21397861
- Jimenez-Vidal M, Gasol E, Zorzano A, Nunes V, Palacin M, Chillaron J. Thiol modification of cysteine 327 in the eighth transmembrane domain of the light subunit xCT of the heteromeric cystine/glutamate antiporter suggests close proximity to the substrate binding site/permeation pathway.
J Biol Chem 2004; 279: 11214-21. PMID: 14722095
- Kaleeba JA, Berger EA. Kaposi's sarcoma-associated herpesvirus fusion-entry receptor: cystine transporter xCT. Science 2006; 311: 1921-4. PMID: 16574866
- Lewerenz J, Maher P. Basal levels of eIF2alpha phosphorylation determine cellular antioxidant status by regulating ATF4 and xCT expression. J Biol Chem 2009; 284: 1106-15. PMID: 19017641
- Liu XX, Li XJ, Zhang B, Liang YJ, Zhou CX, Cao DX, He M, Chen GQ, He JR, Zhao Q.
MicroRNA-26b is underexpressed in human breast cancer and induces cell apoptosis by targeting SLC7A11. FEBS Lett 2011; 585: 1363-7. PMID: 21510944
- Nabeyama A, Kurita A, Asano K, Miyake Y, Yasuda T, Miura I, Nishitai G, Arakawa S, Shimizu S, Wakana S, Yoshida H, Tanaka M. xCT deficiency accelerates chemically induced tumorigenesis. Proc Natl Acad Sci U S A 2010; 107: 6436-41.
PMID: 20308543
- Narang VS, Pauletti GM, Gout PW, Buckley DJ, Buckley AR. Suppression of cystine uptake by sulfasalazine inhibits proliferation of human mammary carcinoma cells.
Anticancer Res 2003; 23: 4571-9.
PMID: 14981898
- Rappocciolo G, Hensler HR, Jais M, Reinhart TA, Pegu A, Jenkins FJ, Rinaldo CR. Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC-SIGN. J Virol 2008; 82: 4793-806.
PMID: 18337571
- Qiang W, Cahill JM, Liu J, Kuang X, Liu N, Scofield VL, Voorhees JR, Reid AJ, Yan M, Lynn WS, Wong PK. Activation of transcription factor Nrf-2 and its downstream targets in response to moloney murine leukemia virus ts1-induced thiol depletion and oxidative stress in astrocytes. J Virol 2004; 78: 11926-38.
PMID: 15479833
- Qiao HX, Hao CJ, Li Y, He X, Chen RS, Cui J, Xu ZH, Li W. JNK activation mediates the apoptosis of xCT-deficient cells. Biochem Biophys Res Commun 2008; 370: 584-8. PMID: 18395005
- Qin Z, Freitas E, Sullivan R, Mohan S, Bacelieri R, Branch D, Romano M, Kearney P, Oates J, Plaisance K, Renne R, Kaleeba J, Parsons C. Upregulation of xCT by KSHV-encoded microRNAs facilitates KSHV dissemination and persistence in an environment of oxidative stress. PLoS Pathog 2010; 6: e1000742. PMID: 20126446
- Sakakura Y, Sato H, Shiiya A, Tamba M, Sagara J, Matsuda M, Okamura N, Makino N, Bannai S.Expression and function of cystine/glutamate transporter in neutrophils. J Leukoc Biol 2007; 81: 974-82. PMID: 17200146
- Sasaki H, Sato H, Kuriyama-Matsumura K, Sato K, Maebara K, Wang H, Tamba M, Itoh K, Yamamoto M, Bannai S. Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J Biol Chem 2002; 277: 44765-71. PMID: 12235164
- Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins.
J Biol Chem 1999; 274:11455-8. PMID: 10206947
- Sato H, Tamba M, Kuriyama-Matsumura K, Okuno S, Bannai S. Molecular cloning and expression of human xCT, the light chain of amino acid transport system xc-.
Antioxid Redox Signal. 2000; 2:665-71. PMID: 11213471
- Sato H, Tamba M, Okuno S, Sato K, Keino-Masu K, Masu M, Bannai S. Distribution of cystine/glutamate exchange transporter, system x(c)-, in the mouse brain.
J Neurosci 2002; 22: 8028-33.PMID: 12223556
- Sato H, Nomura S, Maebara K, Sato K, Tamba M, Bannai S. Transcriptional control of cystine/glutamate transporter gene by amino acid deprivation.
Biochem Biophys Res Commun 2004; 325: 109-16. PMID: 155522208
- Savaskan NE, Heckel A, Hahnen E, Engelhorn T, Doerfler A, Ganslandt O, Nimsky C, Buchfelder M, Ey¨ąpoglu IY. Small interfering RNA-mediated xCT silencing in gliomas inhibits neurodegeneration and alleviates brain edema. Nat Med 2008;14: 629-32.PMID: 18469825
- Veettil MV, Sharma-Walia N, Sadagopan S, Raghu H, Sivakumar R, Naranatt PP, Chandran B. RhoA-GTPase facilitates entry of Kaposi's sarcoma-associated herpesvirus into adherent target cells in a Src-dependent manner.
J Virol 2006; 80: 11432-46. PMID: 17005646
- Veettil MV, Sadagopan S, Sharma-Walia N, Wang FZ, Raghu H, Varga L, Chandran B. Kaposi's sarcoma-associated herpesvirus forms a multimolecular complex of integrins (alphaVbeta5, alphaVbeta3, and alpha3beta1) and CD98-xCT during infection of human dermal microvascular endothelial cells, and CD98-xCT is essential for the postentry stage of infection. J Virol 2008; 82: 12126-44. PMID: 18829766
- Wang H, Tamba M, Kimata M, Sakamoto K, Bannai S, Sato H. Expression of the activity of cystine/glutamate exchange transporter, system x(c)(-), by xCT and rBAT.
Biochem Biophys Res Commun 2003; 305: 611-8. PMID: 12763038
- Zeng Y, Li Y, Chen RS, He X, Yang L, Li W. Overexpression of xCT induces up-regulation of 14-3-3beta in Kaposi's sarcoma. Biosci Rep 2010; 30: 277-83. PMID: 20100173
EDIT HISTORY:
Created by Wei Li, 08/18/2005
Updated by Wei Li, 04/06/2006
Updated by Wei Li, 12/28/2006
Updated by Wei Li, 08/20/2008
Updated by Wei Li, 03/12/2009
Updated by Wei Li, 05/29/2011
Tissue specificity: Widely expressed. It is expressed in Muller and retinal pigment epithelial cells, and a retinal ganglion cell line (RGC-5). Oxidative stress upregulates this transport system in RGC-5 cells (Dun, et al). Northern blot analysis demonstrated that the expression of both xCT and 4F2hc was significantly enhanced by oxygen. (Sato, et al (2000)).

BMR: Bone marrow; SPL: Spleen; TMS: Thymus; BRN: Brain; SPC: Spinal cord; HRT: Heart; MSL: Skeletal muscle; LVR; Liver; PNC: Pancreas; PST: Prostate; KDN: Kidney; LNG: Lung. (data from GeneCards )
PROTEIN
Sequence
xCT (NP_055146): 501aa, UniProtKB/Swiss-Prot entry Q9UPY5.
Synonym: Amino acid transport system xc-, Calcium channel blocker resistance protein CCBR1, Cystine/glutamate transporter, solute carrier family 7 (cationic amino acid transporter, y+ system) member 11
Ortholog
Species
Mouse Rat Dog Fowl GeneView
Slc7a11
Slc7a11
LOC483821
LOC428731
Protein
NP_036120 (502aa)
XP_227120 (502aa)
XP_540941 (551aa)
XP_426289 (580aa)
Identities
89% /500aa 87% /500aa 92% /482aa 68% /578aa
View multiple sequence alignment (PDF file) by ClustalW and GeneDoc.
Domain
(1) Domains predicted by SMART:
AA_permease: 48- 479
(2) Transmembrane domains predicted by SOSUI:
This amino acid sequence is of a MEMBRANE PROTEIN which have 13 transmembrane helices.
No. N terminal transmembrane region C terminal type length
1 46 LLRGVSIIIGTIIGAGIFISPKG 68 PRIMARY 23
2 75 SVGMSLTIWTVCGVLSLFGALSY 97 PRIMARY 23
3 109 GHYTYILEVFGPLPAFVRVWVEL 131 SECONDARY 23
4 136 PAATAVISLAFGRYILEPFFIQC 158 SECONDARY 23
5 162 ELAIKLITAVGITVVMVLNSMSV 184 PRIMARY 23
6 192 IFLTFCKLTAILIIIVPGVMQLI 214 PRIMARY 23
7 235 LPLAFYYGMYAYAGWFYLNFVTE 257 SECONDARY 23
8 268 LAICISMAIVTIGYVLTNVAYFT 290 PRIMARY 23
9 298 LLSNAVAVTFSERLLGNFSLAVP 320 SECONDARY 23
10 363 HTPLPAVIVLHPLTMIMLFSGDL 385 SECONDARY 23
11 389 LNFLSFARWLFIGLAVAGLIYLR 411 PRIMARY 23
12 422 KVPLFIPALFSFTCLFMVALSLY 444 PRIMARY 23
13 450 TGIGFVITLTGVPAYYLFIIWD 471 SECONDARY 22
Note: a topological model for xCT of 12 transmembrane domains with the N and C termini located inside the cell was proposed by Gasel et al .
(3) Graphic view of InterPro domain structure.
Motif/Site
(1) Predicted results by ScanProsite:
a) N-glycosylation site: 314 to 317 NFSL.
b) cAMP- and cGMP-dependent protein kinase phosphorylation site: 42 to 45 RKVT; 361 to 364 RKHT.
c) Protein kinase C phosphorylation site: 65 to 67 SPK; 103 to 105 TIK; 187 to 189 SAR; 226 to 228 SGR; 308 to 310 SER; 481 to 483 SEK.
d) Casein kinase II phosphorylation site: 96 to 99 SYAE; 226 to 229 SGRD; 306 to 309 TFSE.
e) N-myristoylation site: 9 sites.
(2) Predicted results of subprograms by PSORT II:
a) N-terminal signal peptide: none
b) 11 tentative TMs, membrane topology: type 3a
c) KDEL ER retention motif in C-terminus: none
d) ER membrane retention signals:
XXRR-like motif in the N-terminus: VRKP; KKXX-like motif in the C-terminus: EEDK
e) VAC possible vacuolar targeting motif: none
f) Actinin-type actin-binding motif: type 1: none; type 2: none
g) Prenylation motif: none
h) memYQRL transport motif from cell surface to Golgi: none
i) Tyrosines in the tail: none
j) Dileucine motif in the tail: none
3D Model
(1)ModBase predicted comparative 3D structure of Q9UPY5 from UCSC Genome Sorter.



From left to right: Front, Top, and Side views of predicted protein.
2D-PAGE
This protein does not exist in the current release of SWISS-2DPAGE.
Computed theoretical MW=55,423Da, pI=9.29 (NP_055146).
FUNCTION
Ontology
(1) Biological process: amino acid transport.
(2) Pheomelanin production.
(3) Oxidative stress.
(4) Belongs to the amino acid-polyamine-organocation (APC) superfamily. L-type amino acid transporter (LAT) (TC 2.A.3.8) family.
Location
Integral membrane protein. Transport system xc- was found in plasma membrane of cultured mammalian cells (Sato, et al (1999)).
Interaction
SLC7A11 (xCT), together with SLC3A2 (4F2hc, or CD98) linked by a disulfide bond at residue C158, encodes the heterodimeric amino acid transport system x(c)(-), which mediates cystine-glutamate exchange and thereby regulates intracellular glutathione levels. System x(c)- activity is important to maintain both intracellular glutathione levels and the redox balance between cystine and cysteine in the extracellular milieu. rBAT can replace 4F2hc in the expression of the activity of system x(c)(-) (Wang, et al).
In mouse NIH3T3 cells, the activity of system x(c)(-) and xCT mRNA is induced not only by deprivation of cystine but also by deprivation of other amino acids via a genomic cis-element termed amino acid response element (AARE) (Sato, et al (2004)). Overexpression of Nrf-2 in astrocytes specifically increases expression of the xCT cystine/glutamate antiporter and other thiol synthesis-related proteins, to promote cell survival (Qiang, et al). Cys(327) is a functionally important residue accessible to the aqueous extracellular environment and is structurally linked to the permeation pathway and/or the substrate binding site (Jimenez-Vidal, et al).
A CD44 variant (CD44v) interacts with xCT and controls the intracellular level of reduced glutathione (GSH). Ablation of CD44 induced loss of xCT from the cell surface and suppressed tumor growth in a transgenic mouse model of gastric cancer. It also induced activation of p38(MAPK), a downstream target of ROS, and expression of the gene for the cell cycle inhibitor p21(CIP1/WAF1)(Ishimoto, et al). xCT has been identified as a receptor mediating KSHV cell fusion (Kaleeba, et al).
miR-26b, which is down-regulated in human breast cancer specimens and cell lines, impairs viability and triggers apoptosis of human breast cancer MCF7 cells. SLC7A11 is identified as a direct target of miR-26b and its expression is remarkably increased in both breast cancer cell lines and clinical samples (Liu, et al).
Pathway
System x(c)(-) is a sodium-independent, high-affinity transporter of anionic amino acids with high specificity for anionic form of cystine and glutamate. Chintala, et al have reported that Slc7a11 regulates the pheomelanin production and cell proliferation. In melanosomes, dopaquinone reacts with cysteine to yield cysteinldopa, which is a precursor of pheomelanin. Cysteine is a component of the tripeptide glutathione. Glutathione metabolism pathway is described in KEGG Pathways.
MUTATION
Allele or SNP
No mutation has been reported in 32 human HPS patients who lack mutations in the seven known HPS genes (Chintala, et al).
SNPs deposited in dbSNP.
Distribution
(none)
Effect
(none)
PHENOTYPE
SLC7A11 is described in OMIM 607933.
Cystine uptake in human cancer cells is largely mediated by system Xc. xCT is relatively highly expressed in lung, colon, and central nervous system cancer cells (Huang, et al). Modulation of xCT activity is possiblely untilized in cancer therapy. Pharmacologic inhibition of system Xc remarkably inhibits the proliferation of several types of cancer cells, including lymphoma, glioma, prostate and breast cancer (Chung, et al; Doxsee, et al; Gout, et al; Narang, et al). This is thought be mediated by the depletion of GSH, which results in an increase of reactive oxygen species (ROS), and ultimately leads to caspase-dependent apoptosis (Doxsee, et al; Sakakura, et al). The apoptosis is likely mediated by c-Jun N-terminal kinase (JNK) activation. The JNK activation triggers both a caspase-dependent (caspases-9 and -3) and an ER stress-mediated (eIF2 and CHOP) pathway to induce apoptosis (Qiao, et al). In the absence of phospho-eIF2alpha, xCT expression is down-regulated that is mediated by nuclear factor ATF4, implicating the role eIF2alpha-ATF4-xCT module in neurodegeneration (Lewerenz, et al).
Disruption of xCT enhanced homotypic cell-cell adhesion and attenuated cell-extracellular matrix adhesion in an esophageal cancer cell line, KYSE150. SASP significantly inhibited both cell invasion of KYSE150 in vitro and its experimental metastasis in nude mice. Caveolin-1 was upregulated and beta-catenin was recruited to the plasma membrane when xCT was deficient, which were followed by the inhibition of beta-catenin transcriptional activity. Upregulation of caveolin-1 and inhibition of tumor cell invasion were mediated by reactive oxygen species-induced p38 MAPK activation (Chen, et al). Savaskan et al show that genetic or pharmacological inhibition of the glutamate transporter Xc system in gliomas leads to abrogated neurodegeneration, attenuated perifocal edema and prolonged survival. xCT deficiency causes unremitting inflammation because of the impaired survival of activated macrophages at the inflammatory site (Nabeyama, et al).
Kaposi's sarcoma-associated herpesvirus (KSHV, human herpesvirus 8) is the causative agent of Kaposi's sarcoma and other lymphoproliferative syndromes often associated with HIV/AIDS. xCT mediates the entry of KSHV which implicates its involvement in the infectious process of this pathogen (Kaleeba, et al; Veettil, et al (2006); Rappocciolo, et al). KSHV interacts with functionally related integrins (alphaVbeta3, alpha3beta1, and alphaVbeta5) and CD98/xCT molecules in a temporal fashion to form a multimolecular complex during the early stages of endothelial cell infection, probably mediating multiple roles in entry, signal transduction, and viral-gene expression (Veettil, et al (2008)). xCT was overexpressed in KS tissues and HHV-8-positive BCBL-1 cells. 14-3-3beta is a downstream effector of xCT in KS to mediate the cell proliferation (Zeng, et al). KSHV-encoded microRNAs (KSHV miRNAs) upregulate xCT expression by macrophages and endothelial cells, largely through miR-K12-11 suppression of BACH-1-a negative regulator of transcription recognizing antioxidant response elements within gene promoters (Qin, et al).
REFERENCE
- Chen RS, Song YM, Zhou ZY, Tong T, Li Y, Fu M, Guo XL, Dong LJ, He X, Qiao HX, Zhan QM, Li W. Disruption of xCT inhibits cancer cell metastasis via the caveolin-1/beta-catenin pathway. Oncogene 2009; 28: 599-609. PMID: 19015640
- Chintala S, Li W, Lamoreux ML, Ito S, Wakamatsu K, Sviderskaya EV, Bennett DC, Park YM, Gahl WA, Huizing M, Spritz RA, Ben S, Novak EK, Tan J, Swank RT.
Slc7a11 gene controls production of pheomelanin pigment and proliferation of cultured cells. Proc Natl Acad Sci U S A 2005; 102: 10964-9. PMID: 16037214
- Chung WJ, Lyons SA, Nelson GM, Hamza H, Gladson CL, Gillespie GY, Sontheimer H. Inhibition of cystine uptake disrupts the growth of primary brain tumors.
J Neurosci 2005; 25: 7101-10.
PMID: 16079392
- Doxsee DW, Gout PW, Kurita T, Lo M, Buckley AR, Wang Y, Xue H, Karp CM, Cutz JC, Cunha GR, Wang YZ. Sulfasalazine-induced cystine starvation: potential use for prostate cancer therapy. Prostate 2007; 67: 162-71.
PMID: 17075799
- Dun Y, Mysona B, Van Ells T, Amarnath L, Shamsul Ola M, Ganapathy V, Smith SB. Expression of the cystine-glutamate exchanger (x(c) (-)) in retinal ganglion cells and regulation by nitric oxide and oxidative stress. Cell Tissue Res 2006; 324: 189-202. PMID: 16444546
- Gasol E, Jimenez-Vidal M, Chillaron J, Zorzano A, Palacin M. Membrane topology of system xc- light subunit reveals a re-entrant loop with substrate-restricted accessibility.
J Biol Chem 2004; 279: 31228-36. PMID: 15151999
- Gout PW, Buckley AR, Simms CR, Bruchovsky N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the x(c)- cystine transporter: a new action for an old drug. Leukemia. 2001; 15: 1633-40.PMID: 11587223
- Huang Y, Dai Z, Barbacioru C, Sad¨¦e W. Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity and chemoresistance.Cancer Res 2005; 65: 7446-54.
PMID: 16103098
- Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, Masuko T, Shimizu T, Ishikawa T, Kai K, Takahashi E, Imamura Y, Baba Y, Ohmura M, Suematsu M, Baba H, Saya H. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 2011; 19: 387-400. PMID: 21397861
- Jimenez-Vidal M, Gasol E, Zorzano A, Nunes V, Palacin M, Chillaron J. Thiol modification of cysteine 327 in the eighth transmembrane domain of the light subunit xCT of the heteromeric cystine/glutamate antiporter suggests close proximity to the substrate binding site/permeation pathway.
J Biol Chem 2004; 279: 11214-21. PMID: 14722095
- Kaleeba JA, Berger EA. Kaposi's sarcoma-associated herpesvirus fusion-entry receptor: cystine transporter xCT. Science 2006; 311: 1921-4. PMID: 16574866
- Lewerenz J, Maher P. Basal levels of eIF2alpha phosphorylation determine cellular antioxidant status by regulating ATF4 and xCT expression. J Biol Chem 2009; 284: 1106-15. PMID: 19017641
- Liu XX, Li XJ, Zhang B, Liang YJ, Zhou CX, Cao DX, He M, Chen GQ, He JR, Zhao Q.
MicroRNA-26b is underexpressed in human breast cancer and induces cell apoptosis by targeting SLC7A11. FEBS Lett 2011; 585: 1363-7. PMID: 21510944
- Nabeyama A, Kurita A, Asano K, Miyake Y, Yasuda T, Miura I, Nishitai G, Arakawa S, Shimizu S, Wakana S, Yoshida H, Tanaka M. xCT deficiency accelerates chemically induced tumorigenesis. Proc Natl Acad Sci U S A 2010; 107: 6436-41.
PMID: 20308543
- Narang VS, Pauletti GM, Gout PW, Buckley DJ, Buckley AR. Suppression of cystine uptake by sulfasalazine inhibits proliferation of human mammary carcinoma cells.
Anticancer Res 2003; 23: 4571-9.
PMID: 14981898
- Rappocciolo G, Hensler HR, Jais M, Reinhart TA, Pegu A, Jenkins FJ, Rinaldo CR. Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC-SIGN. J Virol 2008; 82: 4793-806.
PMID: 18337571
- Qiang W, Cahill JM, Liu J, Kuang X, Liu N, Scofield VL, Voorhees JR, Reid AJ, Yan M, Lynn WS, Wong PK. Activation of transcription factor Nrf-2 and its downstream targets in response to moloney murine leukemia virus ts1-induced thiol depletion and oxidative stress in astrocytes. J Virol 2004; 78: 11926-38.
PMID: 15479833
- Qiao HX, Hao CJ, Li Y, He X, Chen RS, Cui J, Xu ZH, Li W. JNK activation mediates the apoptosis of xCT-deficient cells. Biochem Biophys Res Commun 2008; 370: 584-8. PMID: 18395005
- Qin Z, Freitas E, Sullivan R, Mohan S, Bacelieri R, Branch D, Romano M, Kearney P, Oates J, Plaisance K, Renne R, Kaleeba J, Parsons C. Upregulation of xCT by KSHV-encoded microRNAs facilitates KSHV dissemination and persistence in an environment of oxidative stress. PLoS Pathog 2010; 6: e1000742. PMID: 20126446
- Sakakura Y, Sato H, Shiiya A, Tamba M, Sagara J, Matsuda M, Okamura N, Makino N, Bannai S.Expression and function of cystine/glutamate transporter in neutrophils. J Leukoc Biol 2007; 81: 974-82. PMID: 17200146
- Sasaki H, Sato H, Kuriyama-Matsumura K, Sato K, Maebara K, Wang H, Tamba M, Itoh K, Yamamoto M, Bannai S. Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J Biol Chem 2002; 277: 44765-71. PMID: 12235164
- Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins.
J Biol Chem 1999; 274:11455-8. PMID: 10206947
- Sato H, Tamba M, Kuriyama-Matsumura K, Okuno S, Bannai S. Molecular cloning and expression of human xCT, the light chain of amino acid transport system xc-.
Antioxid Redox Signal. 2000; 2:665-71. PMID: 11213471
- Sato H, Tamba M, Okuno S, Sato K, Keino-Masu K, Masu M, Bannai S. Distribution of cystine/glutamate exchange transporter, system x(c)-, in the mouse brain.
J Neurosci 2002; 22: 8028-33.PMID: 12223556
- Sato H, Nomura S, Maebara K, Sato K, Tamba M, Bannai S. Transcriptional control of cystine/glutamate transporter gene by amino acid deprivation.
Biochem Biophys Res Commun 2004; 325: 109-16. PMID: 155522208
- Savaskan NE, Heckel A, Hahnen E, Engelhorn T, Doerfler A, Ganslandt O, Nimsky C, Buchfelder M, Ey¨ąpoglu IY. Small interfering RNA-mediated xCT silencing in gliomas inhibits neurodegeneration and alleviates brain edema. Nat Med 2008;14: 629-32.PMID: 18469825
- Veettil MV, Sharma-Walia N, Sadagopan S, Raghu H, Sivakumar R, Naranatt PP, Chandran B. RhoA-GTPase facilitates entry of Kaposi's sarcoma-associated herpesvirus into adherent target cells in a Src-dependent manner.
J Virol 2006; 80: 11432-46. PMID: 17005646
- Veettil MV, Sadagopan S, Sharma-Walia N, Wang FZ, Raghu H, Varga L, Chandran B. Kaposi's sarcoma-associated herpesvirus forms a multimolecular complex of integrins (alphaVbeta5, alphaVbeta3, and alpha3beta1) and CD98-xCT during infection of human dermal microvascular endothelial cells, and CD98-xCT is essential for the postentry stage of infection. J Virol 2008; 82: 12126-44. PMID: 18829766
- Wang H, Tamba M, Kimata M, Sakamoto K, Bannai S, Sato H. Expression of the activity of cystine/glutamate exchange transporter, system x(c)(-), by xCT and rBAT.
Biochem Biophys Res Commun 2003; 305: 611-8. PMID: 12763038
- Zeng Y, Li Y, Chen RS, He X, Yang L, Li W. Overexpression of xCT induces up-regulation of 14-3-3beta in Kaposi's sarcoma. Biosci Rep 2010; 30: 277-83. PMID: 20100173
EDIT HISTORY:
Created by Wei Li, 08/18/2005
Updated by Wei Li, 04/06/2006
Updated by Wei Li, 12/28/2006
Updated by Wei Li, 08/20/2008
Updated by Wei Li, 03/12/2009
Updated by Wei Li, 05/29/2011
xCT (NP_055146): 501aa, UniProtKB/Swiss-Prot entry Q9UPY5.
Synonym: Amino acid transport system xc-, Calcium channel blocker resistance protein CCBR1, Cystine/glutamate transporter, solute carrier family 7 (cationic amino acid transporter, y+ system) member 11
Ortholog
Species
Mouse Rat Dog Fowl GeneView
Slc7a11
Slc7a11
LOC483821
LOC428731
Protein
NP_036120 (502aa)
XP_227120 (502aa)
XP_540941 (551aa)
XP_426289 (580aa)
Identities
89% /500aa 87% /500aa 92% /482aa 68% /578aa
View multiple sequence alignment (PDF file) by ClustalW and GeneDoc.
Domain
(1) Domains predicted by SMART:
AA_permease: 48- 479
(2) Transmembrane domains predicted by SOSUI:
This amino acid sequence is of a MEMBRANE PROTEIN which have 13 transmembrane helices.
No. N terminal transmembrane region C terminal type length
1 46 LLRGVSIIIGTIIGAGIFISPKG 68 PRIMARY 23
2 75 SVGMSLTIWTVCGVLSLFGALSY 97 PRIMARY 23
3 109 GHYTYILEVFGPLPAFVRVWVEL 131 SECONDARY 23
4 136 PAATAVISLAFGRYILEPFFIQC 158 SECONDARY 23
5 162 ELAIKLITAVGITVVMVLNSMSV 184 PRIMARY 23
6 192 IFLTFCKLTAILIIIVPGVMQLI 214 PRIMARY 23
7 235 LPLAFYYGMYAYAGWFYLNFVTE 257 SECONDARY 23
8 268 LAICISMAIVTIGYVLTNVAYFT 290 PRIMARY 23
9 298 LLSNAVAVTFSERLLGNFSLAVP 320 SECONDARY 23
10 363 HTPLPAVIVLHPLTMIMLFSGDL 385 SECONDARY 23
11 389 LNFLSFARWLFIGLAVAGLIYLR 411 PRIMARY 23
12 422 KVPLFIPALFSFTCLFMVALSLY 444 PRIMARY 23
13 450 TGIGFVITLTGVPAYYLFIIWD 471 SECONDARY 22
Note: a topological model for xCT of 12 transmembrane domains with the N and C termini located inside the cell was proposed by Gasel et al .
(3) Graphic view of InterPro domain structure.
Motif/Site
(1) Predicted results by ScanProsite:
a) N-glycosylation site: 314 to 317 NFSL.
b) cAMP- and cGMP-dependent protein kinase phosphorylation site: 42 to 45 RKVT; 361 to 364 RKHT.
c) Protein kinase C phosphorylation site: 65 to 67 SPK; 103 to 105 TIK; 187 to 189 SAR; 226 to 228 SGR; 308 to 310 SER; 481 to 483 SEK.
d) Casein kinase II phosphorylation site: 96 to 99 SYAE; 226 to 229 SGRD; 306 to 309 TFSE.
e) N-myristoylation site: 9 sites.
(2) Predicted results of subprograms by PSORT II:
a) N-terminal signal peptide: none
b) 11 tentative TMs, membrane topology: type 3a
c) KDEL ER retention motif in C-terminus: none
d) ER membrane retention signals:
XXRR-like motif in the N-terminus: VRKP; KKXX-like motif in the C-terminus: EEDK
e) VAC possible vacuolar targeting motif: none
f) Actinin-type actin-binding motif: type 1: none; type 2: none
g) Prenylation motif: none
h) memYQRL transport motif from cell surface to Golgi: none
i) Tyrosines in the tail: none
j) Dileucine motif in the tail: none
3D Model
(1)ModBase predicted comparative 3D structure of Q9UPY5 from UCSC Genome Sorter.



From left to right: Front, Top, and Side views of predicted protein.
2D-PAGE
This protein does not exist in the current release of SWISS-2DPAGE.
Computed theoretical MW=55,423Da, pI=9.29 (NP_055146).
FUNCTION
Ontology
(1) Biological process: amino acid transport.
(2) Pheomelanin production.
(3) Oxidative stress.
(4) Belongs to the amino acid-polyamine-organocation (APC) superfamily. L-type amino acid transporter (LAT) (TC 2.A.3.8) family.
Location
Integral membrane protein. Transport system xc- was found in plasma membrane of cultured mammalian cells (Sato, et al (1999)).
Interaction
SLC7A11 (xCT), together with SLC3A2 (4F2hc, or CD98) linked by a disulfide bond at residue C158, encodes the heterodimeric amino acid transport system x(c)(-), which mediates cystine-glutamate exchange and thereby regulates intracellular glutathione levels. System x(c)- activity is important to maintain both intracellular glutathione levels and the redox balance between cystine and cysteine in the extracellular milieu. rBAT can replace 4F2hc in the expression of the activity of system x(c)(-) (Wang, et al).
In mouse NIH3T3 cells, the activity of system x(c)(-) and xCT mRNA is induced not only by deprivation of cystine but also by deprivation of other amino acids via a genomic cis-element termed amino acid response element (AARE) (Sato, et al (2004)). Overexpression of Nrf-2 in astrocytes specifically increases expression of the xCT cystine/glutamate antiporter and other thiol synthesis-related proteins, to promote cell survival (Qiang, et al). Cys(327) is a functionally important residue accessible to the aqueous extracellular environment and is structurally linked to the permeation pathway and/or the substrate binding site (Jimenez-Vidal, et al).
A CD44 variant (CD44v) interacts with xCT and controls the intracellular level of reduced glutathione (GSH). Ablation of CD44 induced loss of xCT from the cell surface and suppressed tumor growth in a transgenic mouse model of gastric cancer. It also induced activation of p38(MAPK), a downstream target of ROS, and expression of the gene for the cell cycle inhibitor p21(CIP1/WAF1)(Ishimoto, et al). xCT has been identified as a receptor mediating KSHV cell fusion (Kaleeba, et al).
miR-26b, which is down-regulated in human breast cancer specimens and cell lines, impairs viability and triggers apoptosis of human breast cancer MCF7 cells. SLC7A11 is identified as a direct target of miR-26b and its expression is remarkably increased in both breast cancer cell lines and clinical samples (Liu, et al).
Pathway
System x(c)(-) is a sodium-independent, high-affinity transporter of anionic amino acids with high specificity for anionic form of cystine and glutamate. Chintala, et al have reported that Slc7a11 regulates the pheomelanin production and cell proliferation. In melanosomes, dopaquinone reacts with cysteine to yield cysteinldopa, which is a precursor of pheomelanin. Cysteine is a component of the tripeptide glutathione. Glutathione metabolism pathway is described in KEGG Pathways.
MUTATION
Allele or SNP
No mutation has been reported in 32 human HPS patients who lack mutations in the seven known HPS genes (Chintala, et al).
SNPs deposited in dbSNP.
Distribution
(none)
Effect
(none)
PHENOTYPE
SLC7A11 is described in OMIM 607933.
Cystine uptake in human cancer cells is largely mediated by system Xc. xCT is relatively highly expressed in lung, colon, and central nervous system cancer cells (Huang, et al). Modulation of xCT activity is possiblely untilized in cancer therapy. Pharmacologic inhibition of system Xc remarkably inhibits the proliferation of several types of cancer cells, including lymphoma, glioma, prostate and breast cancer (Chung, et al; Doxsee, et al; Gout, et al; Narang, et al). This is thought be mediated by the depletion of GSH, which results in an increase of reactive oxygen species (ROS), and ultimately leads to caspase-dependent apoptosis (Doxsee, et al; Sakakura, et al). The apoptosis is likely mediated by c-Jun N-terminal kinase (JNK) activation. The JNK activation triggers both a caspase-dependent (caspases-9 and -3) and an ER stress-mediated (eIF2 and CHOP) pathway to induce apoptosis (Qiao, et al). In the absence of phospho-eIF2alpha, xCT expression is down-regulated that is mediated by nuclear factor ATF4, implicating the role eIF2alpha-ATF4-xCT module in neurodegeneration (Lewerenz, et al).
Disruption of xCT enhanced homotypic cell-cell adhesion and attenuated cell-extracellular matrix adhesion in an esophageal cancer cell line, KYSE150. SASP significantly inhibited both cell invasion of KYSE150 in vitro and its experimental metastasis in nude mice. Caveolin-1 was upregulated and beta-catenin was recruited to the plasma membrane when xCT was deficient, which were followed by the inhibition of beta-catenin transcriptional activity. Upregulation of caveolin-1 and inhibition of tumor cell invasion were mediated by reactive oxygen species-induced p38 MAPK activation (Chen, et al). Savaskan et al show that genetic or pharmacological inhibition of the glutamate transporter Xc system in gliomas leads to abrogated neurodegeneration, attenuated perifocal edema and prolonged survival. xCT deficiency causes unremitting inflammation because of the impaired survival of activated macrophages at the inflammatory site (Nabeyama, et al).
Kaposi's sarcoma-associated herpesvirus (KSHV, human herpesvirus 8) is the causative agent of Kaposi's sarcoma and other lymphoproliferative syndromes often associated with HIV/AIDS. xCT mediates the entry of KSHV which implicates its involvement in the infectious process of this pathogen (Kaleeba, et al; Veettil, et al (2006); Rappocciolo, et al). KSHV interacts with functionally related integrins (alphaVbeta3, alpha3beta1, and alphaVbeta5) and CD98/xCT molecules in a temporal fashion to form a multimolecular complex during the early stages of endothelial cell infection, probably mediating multiple roles in entry, signal transduction, and viral-gene expression (Veettil, et al (2008)). xCT was overexpressed in KS tissues and HHV-8-positive BCBL-1 cells. 14-3-3beta is a downstream effector of xCT in KS to mediate the cell proliferation (Zeng, et al). KSHV-encoded microRNAs (KSHV miRNAs) upregulate xCT expression by macrophages and endothelial cells, largely through miR-K12-11 suppression of BACH-1-a negative regulator of transcription recognizing antioxidant response elements within gene promoters (Qin, et al).
REFERENCE
- Chen RS, Song YM, Zhou ZY, Tong T, Li Y, Fu M, Guo XL, Dong LJ, He X, Qiao HX, Zhan QM, Li W. Disruption of xCT inhibits cancer cell metastasis via the caveolin-1/beta-catenin pathway. Oncogene 2009; 28: 599-609. PMID: 19015640
- Chintala S, Li W, Lamoreux ML, Ito S, Wakamatsu K, Sviderskaya EV, Bennett DC, Park YM, Gahl WA, Huizing M, Spritz RA, Ben S, Novak EK, Tan J, Swank RT.
Slc7a11 gene controls production of pheomelanin pigment and proliferation of cultured cells. Proc Natl Acad Sci U S A 2005; 102: 10964-9. PMID: 16037214
- Chung WJ, Lyons SA, Nelson GM, Hamza H, Gladson CL, Gillespie GY, Sontheimer H. Inhibition of cystine uptake disrupts the growth of primary brain tumors.
J Neurosci 2005; 25: 7101-10.
PMID: 16079392
- Doxsee DW, Gout PW, Kurita T, Lo M, Buckley AR, Wang Y, Xue H, Karp CM, Cutz JC, Cunha GR, Wang YZ. Sulfasalazine-induced cystine starvation: potential use for prostate cancer therapy. Prostate 2007; 67: 162-71.
PMID: 17075799
- Dun Y, Mysona B, Van Ells T, Amarnath L, Shamsul Ola M, Ganapathy V, Smith SB. Expression of the cystine-glutamate exchanger (x(c) (-)) in retinal ganglion cells and regulation by nitric oxide and oxidative stress. Cell Tissue Res 2006; 324: 189-202. PMID: 16444546
- Gasol E, Jimenez-Vidal M, Chillaron J, Zorzano A, Palacin M. Membrane topology of system xc- light subunit reveals a re-entrant loop with substrate-restricted accessibility.
J Biol Chem 2004; 279: 31228-36. PMID: 15151999
- Gout PW, Buckley AR, Simms CR, Bruchovsky N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the x(c)- cystine transporter: a new action for an old drug. Leukemia. 2001; 15: 1633-40.PMID: 11587223
- Huang Y, Dai Z, Barbacioru C, Sad¨¦e W. Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity and chemoresistance.Cancer Res 2005; 65: 7446-54.
PMID: 16103098
- Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, Masuko T, Shimizu T, Ishikawa T, Kai K, Takahashi E, Imamura Y, Baba Y, Ohmura M, Suematsu M, Baba H, Saya H. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 2011; 19: 387-400. PMID: 21397861
- Jimenez-Vidal M, Gasol E, Zorzano A, Nunes V, Palacin M, Chillaron J. Thiol modification of cysteine 327 in the eighth transmembrane domain of the light subunit xCT of the heteromeric cystine/glutamate antiporter suggests close proximity to the substrate binding site/permeation pathway.
J Biol Chem 2004; 279: 11214-21. PMID: 14722095
- Kaleeba JA, Berger EA. Kaposi's sarcoma-associated herpesvirus fusion-entry receptor: cystine transporter xCT. Science 2006; 311: 1921-4. PMID: 16574866
- Lewerenz J, Maher P. Basal levels of eIF2alpha phosphorylation determine cellular antioxidant status by regulating ATF4 and xCT expression. J Biol Chem 2009; 284: 1106-15. PMID: 19017641
- Liu XX, Li XJ, Zhang B, Liang YJ, Zhou CX, Cao DX, He M, Chen GQ, He JR, Zhao Q.
MicroRNA-26b is underexpressed in human breast cancer and induces cell apoptosis by targeting SLC7A11. FEBS Lett 2011; 585: 1363-7. PMID: 21510944
- Nabeyama A, Kurita A, Asano K, Miyake Y, Yasuda T, Miura I, Nishitai G, Arakawa S, Shimizu S, Wakana S, Yoshida H, Tanaka M. xCT deficiency accelerates chemically induced tumorigenesis. Proc Natl Acad Sci U S A 2010; 107: 6436-41.
PMID: 20308543
- Narang VS, Pauletti GM, Gout PW, Buckley DJ, Buckley AR. Suppression of cystine uptake by sulfasalazine inhibits proliferation of human mammary carcinoma cells.
Anticancer Res 2003; 23: 4571-9.
PMID: 14981898
- Rappocciolo G, Hensler HR, Jais M, Reinhart TA, Pegu A, Jenkins FJ, Rinaldo CR. Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC-SIGN. J Virol 2008; 82: 4793-806.
PMID: 18337571
- Qiang W, Cahill JM, Liu J, Kuang X, Liu N, Scofield VL, Voorhees JR, Reid AJ, Yan M, Lynn WS, Wong PK. Activation of transcription factor Nrf-2 and its downstream targets in response to moloney murine leukemia virus ts1-induced thiol depletion and oxidative stress in astrocytes. J Virol 2004; 78: 11926-38.
PMID: 15479833
- Qiao HX, Hao CJ, Li Y, He X, Chen RS, Cui J, Xu ZH, Li W. JNK activation mediates the apoptosis of xCT-deficient cells. Biochem Biophys Res Commun 2008; 370: 584-8. PMID: 18395005
- Qin Z, Freitas E, Sullivan R, Mohan S, Bacelieri R, Branch D, Romano M, Kearney P, Oates J, Plaisance K, Renne R, Kaleeba J, Parsons C. Upregulation of xCT by KSHV-encoded microRNAs facilitates KSHV dissemination and persistence in an environment of oxidative stress. PLoS Pathog 2010; 6: e1000742. PMID: 20126446
- Sakakura Y, Sato H, Shiiya A, Tamba M, Sagara J, Matsuda M, Okamura N, Makino N, Bannai S.Expression and function of cystine/glutamate transporter in neutrophils. J Leukoc Biol 2007; 81: 974-82. PMID: 17200146
- Sasaki H, Sato H, Kuriyama-Matsumura K, Sato K, Maebara K, Wang H, Tamba M, Itoh K, Yamamoto M, Bannai S. Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J Biol Chem 2002; 277: 44765-71. PMID: 12235164
- Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins.
J Biol Chem 1999; 274:11455-8. PMID: 10206947
- Sato H, Tamba M, Kuriyama-Matsumura K, Okuno S, Bannai S. Molecular cloning and expression of human xCT, the light chain of amino acid transport system xc-.
Antioxid Redox Signal. 2000; 2:665-71. PMID: 11213471
- Sato H, Tamba M, Okuno S, Sato K, Keino-Masu K, Masu M, Bannai S. Distribution of cystine/glutamate exchange transporter, system x(c)-, in the mouse brain.
J Neurosci 2002; 22: 8028-33.PMID: 12223556
- Sato H, Nomura S, Maebara K, Sato K, Tamba M, Bannai S. Transcriptional control of cystine/glutamate transporter gene by amino acid deprivation.
Biochem Biophys Res Commun 2004; 325: 109-16. PMID: 155522208
- Savaskan NE, Heckel A, Hahnen E, Engelhorn T, Doerfler A, Ganslandt O, Nimsky C, Buchfelder M, Ey¨ąpoglu IY. Small interfering RNA-mediated xCT silencing in gliomas inhibits neurodegeneration and alleviates brain edema. Nat Med 2008;14: 629-32.PMID: 18469825
- Veettil MV, Sharma-Walia N, Sadagopan S, Raghu H, Sivakumar R, Naranatt PP, Chandran B. RhoA-GTPase facilitates entry of Kaposi's sarcoma-associated herpesvirus into adherent target cells in a Src-dependent manner.
J Virol 2006; 80: 11432-46. PMID: 17005646
- Veettil MV, Sadagopan S, Sharma-Walia N, Wang FZ, Raghu H, Varga L, Chandran B. Kaposi's sarcoma-associated herpesvirus forms a multimolecular complex of integrins (alphaVbeta5, alphaVbeta3, and alpha3beta1) and CD98-xCT during infection of human dermal microvascular endothelial cells, and CD98-xCT is essential for the postentry stage of infection. J Virol 2008; 82: 12126-44. PMID: 18829766
- Wang H, Tamba M, Kimata M, Sakamoto K, Bannai S, Sato H. Expression of the activity of cystine/glutamate exchange transporter, system x(c)(-), by xCT and rBAT.
Biochem Biophys Res Commun 2003; 305: 611-8. PMID: 12763038
- Zeng Y, Li Y, Chen RS, He X, Yang L, Li W. Overexpression of xCT induces up-regulation of 14-3-3beta in Kaposi's sarcoma. Biosci Rep 2010; 30: 277-83. PMID: 20100173
EDIT HISTORY:
Created by Wei Li, 08/18/2005
Updated by Wei Li, 04/06/2006
Updated by Wei Li, 12/28/2006
Updated by Wei Li, 08/20/2008
Updated by Wei Li, 03/12/2009
Updated by Wei Li, 05/29/2011
| Species | Mouse | Rat | Dog | Fowl |
| GeneView | Slc7a11 | Slc7a11 | LOC483821 | LOC428731 |
| Protein | NP_036120 (502aa) | XP_227120 (502aa) | XP_540941 (551aa) | XP_426289 (580aa) |
| Identities | 89% /500aa | 87% /500aa | 92% /482aa | 68% /578aa |
View multiple sequence alignment (PDF file) by ClustalW and GeneDoc.
Domain
(1) Domains predicted by SMART:
AA_permease: 48- 479
(2) Transmembrane domains predicted by SOSUI:
This amino acid sequence is of a MEMBRANE PROTEIN which have 13 transmembrane helices.
No. N terminal transmembrane region C terminal type length
1 46 LLRGVSIIIGTIIGAGIFISPKG 68 PRIMARY 23
2 75 SVGMSLTIWTVCGVLSLFGALSY 97 PRIMARY 23
3 109 GHYTYILEVFGPLPAFVRVWVEL 131 SECONDARY 23
4 136 PAATAVISLAFGRYILEPFFIQC 158 SECONDARY 23
5 162 ELAIKLITAVGITVVMVLNSMSV 184 PRIMARY 23
6 192 IFLTFCKLTAILIIIVPGVMQLI 214 PRIMARY 23
7 235 LPLAFYYGMYAYAGWFYLNFVTE 257 SECONDARY 23
8 268 LAICISMAIVTIGYVLTNVAYFT 290 PRIMARY 23
9 298 LLSNAVAVTFSERLLGNFSLAVP 320 SECONDARY 23
10 363 HTPLPAVIVLHPLTMIMLFSGDL 385 SECONDARY 23
11 389 LNFLSFARWLFIGLAVAGLIYLR 411 PRIMARY 23
12 422 KVPLFIPALFSFTCLFMVALSLY 444 PRIMARY 23
13 450 TGIGFVITLTGVPAYYLFIIWD 471 SECONDARY 22
Note: a topological model for xCT of 12 transmembrane domains with the N and C termini located inside the cell was proposed by Gasel et al .
(3) Graphic view of InterPro domain structure.
Motif/Site
(1) Predicted results by ScanProsite:
a) N-glycosylation site: 314 to 317 NFSL.
b) cAMP- and cGMP-dependent protein kinase phosphorylation site: 42 to 45 RKVT; 361 to 364 RKHT.
c) Protein kinase C phosphorylation site: 65 to 67 SPK; 103 to 105 TIK; 187 to 189 SAR; 226 to 228 SGR; 308 to 310 SER; 481 to 483 SEK.
d) Casein kinase II phosphorylation site: 96 to 99 SYAE; 226 to 229 SGRD; 306 to 309 TFSE.
e) N-myristoylation site: 9 sites.
(2) Predicted results of subprograms by PSORT II:
a) N-terminal signal peptide: none
b) 11 tentative TMs, membrane topology: type 3a
c) KDEL ER retention motif in C-terminus: none
d) ER membrane retention signals:
XXRR-like motif in the N-terminus: VRKP; KKXX-like motif in the C-terminus: EEDK
e) VAC possible vacuolar targeting motif: none
f) Actinin-type actin-binding motif: type 1: none; type 2: none
g) Prenylation motif: none
h) memYQRL transport motif from cell surface to Golgi: none
i) Tyrosines in the tail: none
j) Dileucine motif in the tail: none
3D Model
(1)ModBase predicted comparative 3D structure of Q9UPY5 from UCSC Genome Sorter.



From left to right: Front, Top, and Side views of predicted protein.
2D-PAGE
This protein does not exist in the current release of SWISS-2DPAGE.
Computed theoretical MW=55,423Da, pI=9.29 (NP_055146).
FUNCTION
Ontology
(1) Biological process: amino acid transport.
(2) Pheomelanin production.
(3) Oxidative stress.
(4) Belongs to the amino acid-polyamine-organocation (APC) superfamily. L-type amino acid transporter (LAT) (TC 2.A.3.8) family.
Location
Integral membrane protein. Transport system xc- was found in plasma membrane of cultured mammalian cells (Sato, et al (1999)).
Interaction
SLC7A11 (xCT), together with SLC3A2 (4F2hc, or CD98) linked by a disulfide bond at residue C158, encodes the heterodimeric amino acid transport system x(c)(-), which mediates cystine-glutamate exchange and thereby regulates intracellular glutathione levels. System x(c)- activity is important to maintain both intracellular glutathione levels and the redox balance between cystine and cysteine in the extracellular milieu. rBAT can replace 4F2hc in the expression of the activity of system x(c)(-) (Wang, et al).
In mouse NIH3T3 cells, the activity of system x(c)(-) and xCT mRNA is induced not only by deprivation of cystine but also by deprivation of other amino acids via a genomic cis-element termed amino acid response element (AARE) (Sato, et al (2004)). Overexpression of Nrf-2 in astrocytes specifically increases expression of the xCT cystine/glutamate antiporter and other thiol synthesis-related proteins, to promote cell survival (Qiang, et al). Cys(327) is a functionally important residue accessible to the aqueous extracellular environment and is structurally linked to the permeation pathway and/or the substrate binding site (Jimenez-Vidal, et al).
A CD44 variant (CD44v) interacts with xCT and controls the intracellular level of reduced glutathione (GSH). Ablation of CD44 induced loss of xCT from the cell surface and suppressed tumor growth in a transgenic mouse model of gastric cancer. It also induced activation of p38(MAPK), a downstream target of ROS, and expression of the gene for the cell cycle inhibitor p21(CIP1/WAF1)(Ishimoto, et al). xCT has been identified as a receptor mediating KSHV cell fusion (Kaleeba, et al).
miR-26b, which is down-regulated in human breast cancer specimens and cell lines, impairs viability and triggers apoptosis of human breast cancer MCF7 cells. SLC7A11 is identified as a direct target of miR-26b and its expression is remarkably increased in both breast cancer cell lines and clinical samples (Liu, et al).
Pathway
System x(c)(-) is a sodium-independent, high-affinity transporter of anionic amino acids with high specificity for anionic form of cystine and glutamate. Chintala, et al have reported that Slc7a11 regulates the pheomelanin production and cell proliferation. In melanosomes, dopaquinone reacts with cysteine to yield cysteinldopa, which is a precursor of pheomelanin. Cysteine is a component of the tripeptide glutathione. Glutathione metabolism pathway is described in KEGG Pathways.
MUTATION
Allele or SNP
No mutation has been reported in 32 human HPS patients who lack mutations in the seven known HPS genes (Chintala, et al).
SNPs deposited in dbSNP.
Distribution
(none)
Effect
(none)
PHENOTYPE
SLC7A11 is described in OMIM 607933.
Cystine uptake in human cancer cells is largely mediated by system Xc. xCT is relatively highly expressed in lung, colon, and central nervous system cancer cells (Huang, et al). Modulation of xCT activity is possiblely untilized in cancer therapy. Pharmacologic inhibition of system Xc remarkably inhibits the proliferation of several types of cancer cells, including lymphoma, glioma, prostate and breast cancer (Chung, et al; Doxsee, et al; Gout, et al; Narang, et al). This is thought be mediated by the depletion of GSH, which results in an increase of reactive oxygen species (ROS), and ultimately leads to caspase-dependent apoptosis (Doxsee, et al; Sakakura, et al). The apoptosis is likely mediated by c-Jun N-terminal kinase (JNK) activation. The JNK activation triggers both a caspase-dependent (caspases-9 and -3) and an ER stress-mediated (eIF2 and CHOP) pathway to induce apoptosis (Qiao, et al). In the absence of phospho-eIF2alpha, xCT expression is down-regulated that is mediated by nuclear factor ATF4, implicating the role eIF2alpha-ATF4-xCT module in neurodegeneration (Lewerenz, et al).
Disruption of xCT enhanced homotypic cell-cell adhesion and attenuated cell-extracellular matrix adhesion in an esophageal cancer cell line, KYSE150. SASP significantly inhibited both cell invasion of KYSE150 in vitro and its experimental metastasis in nude mice. Caveolin-1 was upregulated and beta-catenin was recruited to the plasma membrane when xCT was deficient, which were followed by the inhibition of beta-catenin transcriptional activity. Upregulation of caveolin-1 and inhibition of tumor cell invasion were mediated by reactive oxygen species-induced p38 MAPK activation (Chen, et al). Savaskan et al show that genetic or pharmacological inhibition of the glutamate transporter Xc system in gliomas leads to abrogated neurodegeneration, attenuated perifocal edema and prolonged survival. xCT deficiency causes unremitting inflammation because of the impaired survival of activated macrophages at the inflammatory site (Nabeyama, et al).
Kaposi's sarcoma-associated herpesvirus (KSHV, human herpesvirus 8) is the causative agent of Kaposi's sarcoma and other lymphoproliferative syndromes often associated with HIV/AIDS. xCT mediates the entry of KSHV which implicates its involvement in the infectious process of this pathogen (Kaleeba, et al; Veettil, et al (2006); Rappocciolo, et al). KSHV interacts with functionally related integrins (alphaVbeta3, alpha3beta1, and alphaVbeta5) and CD98/xCT molecules in a temporal fashion to form a multimolecular complex during the early stages of endothelial cell infection, probably mediating multiple roles in entry, signal transduction, and viral-gene expression (Veettil, et al (2008)). xCT was overexpressed in KS tissues and HHV-8-positive BCBL-1 cells. 14-3-3beta is a downstream effector of xCT in KS to mediate the cell proliferation (Zeng, et al). KSHV-encoded microRNAs (KSHV miRNAs) upregulate xCT expression by macrophages and endothelial cells, largely through miR-K12-11 suppression of BACH-1-a negative regulator of transcription recognizing antioxidant response elements within gene promoters (Qin, et al).
REFERENCE
- Chen RS, Song YM, Zhou ZY, Tong T, Li Y, Fu M, Guo XL, Dong LJ, He X, Qiao HX, Zhan QM, Li W. Disruption of xCT inhibits cancer cell metastasis via the caveolin-1/beta-catenin pathway. Oncogene 2009; 28: 599-609. PMID: 19015640
- Chintala S, Li W, Lamoreux ML, Ito S, Wakamatsu K, Sviderskaya EV, Bennett DC, Park YM, Gahl WA, Huizing M, Spritz RA, Ben S, Novak EK, Tan J, Swank RT.
Slc7a11 gene controls production of pheomelanin pigment and proliferation of cultured cells. Proc Natl Acad Sci U S A 2005; 102: 10964-9. PMID: 16037214
- Chung WJ, Lyons SA, Nelson GM, Hamza H, Gladson CL, Gillespie GY, Sontheimer H. Inhibition of cystine uptake disrupts the growth of primary brain tumors.
J Neurosci 2005; 25: 7101-10.
PMID: 16079392
- Doxsee DW, Gout PW, Kurita T, Lo M, Buckley AR, Wang Y, Xue H, Karp CM, Cutz JC, Cunha GR, Wang YZ. Sulfasalazine-induced cystine starvation: potential use for prostate cancer therapy. Prostate 2007; 67: 162-71.
PMID: 17075799
- Dun Y, Mysona B, Van Ells T, Amarnath L, Shamsul Ola M, Ganapathy V, Smith SB. Expression of the cystine-glutamate exchanger (x(c) (-)) in retinal ganglion cells and regulation by nitric oxide and oxidative stress. Cell Tissue Res 2006; 324: 189-202. PMID: 16444546
- Gasol E, Jimenez-Vidal M, Chillaron J, Zorzano A, Palacin M. Membrane topology of system xc- light subunit reveals a re-entrant loop with substrate-restricted accessibility.
J Biol Chem 2004; 279: 31228-36. PMID: 15151999
- Gout PW, Buckley AR, Simms CR, Bruchovsky N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the x(c)- cystine transporter: a new action for an old drug. Leukemia. 2001; 15: 1633-40.PMID: 11587223
- Huang Y, Dai Z, Barbacioru C, Sad¨¦e W. Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity and chemoresistance.Cancer Res 2005; 65: 7446-54.
PMID: 16103098
- Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, Masuko T, Shimizu T, Ishikawa T, Kai K, Takahashi E, Imamura Y, Baba Y, Ohmura M, Suematsu M, Baba H, Saya H. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 2011; 19: 387-400. PMID: 21397861
- Jimenez-Vidal M, Gasol E, Zorzano A, Nunes V, Palacin M, Chillaron J. Thiol modification of cysteine 327 in the eighth transmembrane domain of the light subunit xCT of the heteromeric cystine/glutamate antiporter suggests close proximity to the substrate binding site/permeation pathway.
J Biol Chem 2004; 279: 11214-21. PMID: 14722095
- Kaleeba JA, Berger EA. Kaposi's sarcoma-associated herpesvirus fusion-entry receptor: cystine transporter xCT. Science 2006; 311: 1921-4. PMID: 16574866
- Lewerenz J, Maher P. Basal levels of eIF2alpha phosphorylation determine cellular antioxidant status by regulating ATF4 and xCT expression. J Biol Chem 2009; 284: 1106-15. PMID: 19017641
- Liu XX, Li XJ, Zhang B, Liang YJ, Zhou CX, Cao DX, He M, Chen GQ, He JR, Zhao Q.
MicroRNA-26b is underexpressed in human breast cancer and induces cell apoptosis by targeting SLC7A11. FEBS Lett 2011; 585: 1363-7. PMID: 21510944
- Nabeyama A, Kurita A, Asano K, Miyake Y, Yasuda T, Miura I, Nishitai G, Arakawa S, Shimizu S, Wakana S, Yoshida H, Tanaka M. xCT deficiency accelerates chemically induced tumorigenesis. Proc Natl Acad Sci U S A 2010; 107: 6436-41.
PMID: 20308543
- Narang VS, Pauletti GM, Gout PW, Buckley DJ, Buckley AR. Suppression of cystine uptake by sulfasalazine inhibits proliferation of human mammary carcinoma cells.
Anticancer Res 2003; 23: 4571-9.
PMID: 14981898
- Rappocciolo G, Hensler HR, Jais M, Reinhart TA, Pegu A, Jenkins FJ, Rinaldo CR. Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC-SIGN. J Virol 2008; 82: 4793-806.
PMID: 18337571
- Qiang W, Cahill JM, Liu J, Kuang X, Liu N, Scofield VL, Voorhees JR, Reid AJ, Yan M, Lynn WS, Wong PK. Activation of transcription factor Nrf-2 and its downstream targets in response to moloney murine leukemia virus ts1-induced thiol depletion and oxidative stress in astrocytes. J Virol 2004; 78: 11926-38.
PMID: 15479833
- Qiao HX, Hao CJ, Li Y, He X, Chen RS, Cui J, Xu ZH, Li W. JNK activation mediates the apoptosis of xCT-deficient cells. Biochem Biophys Res Commun 2008; 370: 584-8. PMID: 18395005
- Qin Z, Freitas E, Sullivan R, Mohan S, Bacelieri R, Branch D, Romano M, Kearney P, Oates J, Plaisance K, Renne R, Kaleeba J, Parsons C. Upregulation of xCT by KSHV-encoded microRNAs facilitates KSHV dissemination and persistence in an environment of oxidative stress. PLoS Pathog 2010; 6: e1000742. PMID: 20126446
- Sakakura Y, Sato H, Shiiya A, Tamba M, Sagara J, Matsuda M, Okamura N, Makino N, Bannai S.Expression and function of cystine/glutamate transporter in neutrophils. J Leukoc Biol 2007; 81: 974-82. PMID: 17200146
- Sasaki H, Sato H, Kuriyama-Matsumura K, Sato K, Maebara K, Wang H, Tamba M, Itoh K, Yamamoto M, Bannai S. Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J Biol Chem 2002; 277: 44765-71. PMID: 12235164
- Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins.
J Biol Chem 1999; 274:11455-8. PMID: 10206947
- Sato H, Tamba M, Kuriyama-Matsumura K, Okuno S, Bannai S. Molecular cloning and expression of human xCT, the light chain of amino acid transport system xc-.
Antioxid Redox Signal. 2000; 2:665-71. PMID: 11213471
- Sato H, Tamba M, Okuno S, Sato K, Keino-Masu K, Masu M, Bannai S. Distribution of cystine/glutamate exchange transporter, system x(c)-, in the mouse brain.
J Neurosci 2002; 22: 8028-33.PMID: 12223556
- Sato H, Nomura S, Maebara K, Sato K, Tamba M, Bannai S. Transcriptional control of cystine/glutamate transporter gene by amino acid deprivation.
Biochem Biophys Res Commun 2004; 325: 109-16. PMID: 155522208
- Savaskan NE, Heckel A, Hahnen E, Engelhorn T, Doerfler A, Ganslandt O, Nimsky C, Buchfelder M, Ey¨ąpoglu IY. Small interfering RNA-mediated xCT silencing in gliomas inhibits neurodegeneration and alleviates brain edema. Nat Med 2008;14: 629-32.PMID: 18469825
- Veettil MV, Sharma-Walia N, Sadagopan S, Raghu H, Sivakumar R, Naranatt PP, Chandran B. RhoA-GTPase facilitates entry of Kaposi's sarcoma-associated herpesvirus into adherent target cells in a Src-dependent manner.
J Virol 2006; 80: 11432-46. PMID: 17005646
- Veettil MV, Sadagopan S, Sharma-Walia N, Wang FZ, Raghu H, Varga L, Chandran B. Kaposi's sarcoma-associated herpesvirus forms a multimolecular complex of integrins (alphaVbeta5, alphaVbeta3, and alpha3beta1) and CD98-xCT during infection of human dermal microvascular endothelial cells, and CD98-xCT is essential for the postentry stage of infection. J Virol 2008; 82: 12126-44. PMID: 18829766
- Wang H, Tamba M, Kimata M, Sakamoto K, Bannai S, Sato H. Expression of the activity of cystine/glutamate exchange transporter, system x(c)(-), by xCT and rBAT.
Biochem Biophys Res Commun 2003; 305: 611-8. PMID: 12763038
- Zeng Y, Li Y, Chen RS, He X, Yang L, Li W. Overexpression of xCT induces up-regulation of 14-3-3beta in Kaposi's sarcoma. Biosci Rep 2010; 30: 277-83. PMID: 20100173
EDIT HISTORY:
Created by Wei Li, 08/18/2005
Updated by Wei Li, 04/06/2006
Updated by Wei Li, 12/28/2006
Updated by Wei Li, 08/20/2008
Updated by Wei Li, 03/12/2009
Updated by Wei Li, 05/29/2011
(1) Domains predicted by SMART:
AA_permease: 48- 479
(2) Transmembrane domains predicted by SOSUI:
This amino acid sequence is of a MEMBRANE PROTEIN which have 13 transmembrane helices.
| No. | N terminal | transmembrane region | C terminal | type | length |
| 1 | 46 | LLRGVSIIIGTIIGAGIFISPKG | 68 | PRIMARY | 23 |
| 2 | 75 | SVGMSLTIWTVCGVLSLFGALSY | 97 | PRIMARY | 23 |
| 3 | 109 | GHYTYILEVFGPLPAFVRVWVEL | 131 | SECONDARY | 23 |
| 4 | 136 | PAATAVISLAFGRYILEPFFIQC | 158 | SECONDARY | 23 |
| 5 | 162 | ELAIKLITAVGITVVMVLNSMSV | 184 | PRIMARY | 23 |
| 6 | 192 | IFLTFCKLTAILIIIVPGVMQLI | 214 | PRIMARY | 23 |
| 7 | 235 | LPLAFYYGMYAYAGWFYLNFVTE | 257 | SECONDARY | 23 |
| 8 | 268 | LAICISMAIVTIGYVLTNVAYFT | 290 | PRIMARY | 23 |
| 9 | 298 | LLSNAVAVTFSERLLGNFSLAVP | 320 | SECONDARY | 23 |
| 10 | 363 | HTPLPAVIVLHPLTMIMLFSGDL | 385 | SECONDARY | 23 |
| 11 | 389 | LNFLSFARWLFIGLAVAGLIYLR | 411 | PRIMARY | 23 |
| 12 | 422 | KVPLFIPALFSFTCLFMVALSLY | 444 | PRIMARY | 23 |
| 13 | 450 | TGIGFVITLTGVPAYYLFIIWD | 471 | SECONDARY | 22 |
Note: a topological model for xCT of 12 transmembrane domains with the N and C termini located inside the cell was proposed by Gasel et al .
(3) Graphic view of InterPro domain structure.
Motif/Site
(1) Predicted results by ScanProsite:
a) N-glycosylation site: 314 to 317 NFSL.
b) cAMP- and cGMP-dependent protein kinase phosphorylation site: 42 to 45 RKVT; 361 to 364 RKHT.
c) Protein kinase C phosphorylation site: 65 to 67 SPK; 103 to 105 TIK; 187 to 189 SAR; 226 to 228 SGR; 308 to 310 SER; 481 to 483 SEK.
d) Casein kinase II phosphorylation site: 96 to 99 SYAE; 226 to 229 SGRD; 306 to 309 TFSE.
e) N-myristoylation site: 9 sites.
(2) Predicted results of subprograms by PSORT II:
a) N-terminal signal peptide: none
b) 11 tentative TMs, membrane topology: type 3a
c) KDEL ER retention motif in C-terminus: none
d) ER membrane retention signals:
XXRR-like motif in the N-terminus: VRKP; KKXX-like motif in the C-terminus: EEDK
e) VAC possible vacuolar targeting motif: none
f) Actinin-type actin-binding motif: type 1: none; type 2: none
g) Prenylation motif: none
h) memYQRL transport motif from cell surface to Golgi: none
i) Tyrosines in the tail: none
j) Dileucine motif in the tail: none
3D Model
(1)ModBase predicted comparative 3D structure of Q9UPY5 from UCSC Genome Sorter.



From left to right: Front, Top, and Side views of predicted protein.
2D-PAGE
This protein does not exist in the current release of SWISS-2DPAGE.
Computed theoretical MW=55,423Da, pI=9.29 (NP_055146).
FUNCTION
Ontology
(1) Biological process: amino acid transport.
(2) Pheomelanin production.
(3) Oxidative stress.
(4) Belongs to the amino acid-polyamine-organocation (APC) superfamily. L-type amino acid transporter (LAT) (TC 2.A.3.8) family.
Location
Integral membrane protein. Transport system xc- was found in plasma membrane of cultured mammalian cells (Sato, et al (1999)).
Interaction
SLC7A11 (xCT), together with SLC3A2 (4F2hc, or CD98) linked by a disulfide bond at residue C158, encodes the heterodimeric amino acid transport system x(c)(-), which mediates cystine-glutamate exchange and thereby regulates intracellular glutathione levels. System x(c)- activity is important to maintain both intracellular glutathione levels and the redox balance between cystine and cysteine in the extracellular milieu. rBAT can replace 4F2hc in the expression of the activity of system x(c)(-) (Wang, et al).
In mouse NIH3T3 cells, the activity of system x(c)(-) and xCT mRNA is induced not only by deprivation of cystine but also by deprivation of other amino acids via a genomic cis-element termed amino acid response element (AARE) (Sato, et al (2004)). Overexpression of Nrf-2 in astrocytes specifically increases expression of the xCT cystine/glutamate antiporter and other thiol synthesis-related proteins, to promote cell survival (Qiang, et al). Cys(327) is a functionally important residue accessible to the aqueous extracellular environment and is structurally linked to the permeation pathway and/or the substrate binding site (Jimenez-Vidal, et al).
A CD44 variant (CD44v) interacts with xCT and controls the intracellular level of reduced glutathione (GSH). Ablation of CD44 induced loss of xCT from the cell surface and suppressed tumor growth in a transgenic mouse model of gastric cancer. It also induced activation of p38(MAPK), a downstream target of ROS, and expression of the gene for the cell cycle inhibitor p21(CIP1/WAF1)(Ishimoto, et al). xCT has been identified as a receptor mediating KSHV cell fusion (Kaleeba, et al).
miR-26b, which is down-regulated in human breast cancer specimens and cell lines, impairs viability and triggers apoptosis of human breast cancer MCF7 cells. SLC7A11 is identified as a direct target of miR-26b and its expression is remarkably increased in both breast cancer cell lines and clinical samples (Liu, et al).
Pathway
System x(c)(-) is a sodium-independent, high-affinity transporter of anionic amino acids with high specificity for anionic form of cystine and glutamate. Chintala, et al have reported that Slc7a11 regulates the pheomelanin production and cell proliferation. In melanosomes, dopaquinone reacts with cysteine to yield cysteinldopa, which is a precursor of pheomelanin. Cysteine is a component of the tripeptide glutathione. Glutathione metabolism pathway is described in KEGG Pathways.
MUTATION
Allele or SNP
No mutation has been reported in 32 human HPS patients who lack mutations in the seven known HPS genes (Chintala, et al).
SNPs deposited in dbSNP.
Distribution
(none)
Effect
(none)
PHENOTYPE
SLC7A11 is described in OMIM 607933.
Cystine uptake in human cancer cells is largely mediated by system Xc. xCT is relatively highly expressed in lung, colon, and central nervous system cancer cells (Huang, et al). Modulation of xCT activity is possiblely untilized in cancer therapy. Pharmacologic inhibition of system Xc remarkably inhibits the proliferation of several types of cancer cells, including lymphoma, glioma, prostate and breast cancer (Chung, et al; Doxsee, et al; Gout, et al; Narang, et al). This is thought be mediated by the depletion of GSH, which results in an increase of reactive oxygen species (ROS), and ultimately leads to caspase-dependent apoptosis (Doxsee, et al; Sakakura, et al). The apoptosis is likely mediated by c-Jun N-terminal kinase (JNK) activation. The JNK activation triggers both a caspase-dependent (caspases-9 and -3) and an ER stress-mediated (eIF2 and CHOP) pathway to induce apoptosis (Qiao, et al). In the absence of phospho-eIF2alpha, xCT expression is down-regulated that is mediated by nuclear factor ATF4, implicating the role eIF2alpha-ATF4-xCT module in neurodegeneration (Lewerenz, et al).
Disruption of xCT enhanced homotypic cell-cell adhesion and attenuated cell-extracellular matrix adhesion in an esophageal cancer cell line, KYSE150. SASP significantly inhibited both cell invasion of KYSE150 in vitro and its experimental metastasis in nude mice. Caveolin-1 was upregulated and beta-catenin was recruited to the plasma membrane when xCT was deficient, which were followed by the inhibition of beta-catenin transcriptional activity. Upregulation of caveolin-1 and inhibition of tumor cell invasion were mediated by reactive oxygen species-induced p38 MAPK activation (Chen, et al). Savaskan et al show that genetic or pharmacological inhibition of the glutamate transporter Xc system in gliomas leads to abrogated neurodegeneration, attenuated perifocal edema and prolonged survival. xCT deficiency causes unremitting inflammation because of the impaired survival of activated macrophages at the inflammatory site (Nabeyama, et al).
Kaposi's sarcoma-associated herpesvirus (KSHV, human herpesvirus 8) is the causative agent of Kaposi's sarcoma and other lymphoproliferative syndromes often associated with HIV/AIDS. xCT mediates the entry of KSHV which implicates its involvement in the infectious process of this pathogen (Kaleeba, et al; Veettil, et al (2006); Rappocciolo, et al). KSHV interacts with functionally related integrins (alphaVbeta3, alpha3beta1, and alphaVbeta5) and CD98/xCT molecules in a temporal fashion to form a multimolecular complex during the early stages of endothelial cell infection, probably mediating multiple roles in entry, signal transduction, and viral-gene expression (Veettil, et al (2008)). xCT was overexpressed in KS tissues and HHV-8-positive BCBL-1 cells. 14-3-3beta is a downstream effector of xCT in KS to mediate the cell proliferation (Zeng, et al). KSHV-encoded microRNAs (KSHV miRNAs) upregulate xCT expression by macrophages and endothelial cells, largely through miR-K12-11 suppression of BACH-1-a negative regulator of transcription recognizing antioxidant response elements within gene promoters (Qin, et al).
REFERENCE
- Chen RS, Song YM, Zhou ZY, Tong T, Li Y, Fu M, Guo XL, Dong LJ, He X, Qiao HX, Zhan QM, Li W. Disruption of xCT inhibits cancer cell metastasis via the caveolin-1/beta-catenin pathway. Oncogene 2009; 28: 599-609. PMID: 19015640
- Chintala S, Li W, Lamoreux ML, Ito S, Wakamatsu K, Sviderskaya EV, Bennett DC, Park YM, Gahl WA, Huizing M, Spritz RA, Ben S, Novak EK, Tan J, Swank RT.
Slc7a11 gene controls production of pheomelanin pigment and proliferation of cultured cells. Proc Natl Acad Sci U S A 2005; 102: 10964-9. PMID: 16037214
- Chung WJ, Lyons SA, Nelson GM, Hamza H, Gladson CL, Gillespie GY, Sontheimer H. Inhibition of cystine uptake disrupts the growth of primary brain tumors.
J Neurosci 2005; 25: 7101-10.
PMID: 16079392
- Doxsee DW, Gout PW, Kurita T, Lo M, Buckley AR, Wang Y, Xue H, Karp CM, Cutz JC, Cunha GR, Wang YZ. Sulfasalazine-induced cystine starvation: potential use for prostate cancer therapy. Prostate 2007; 67: 162-71.
PMID: 17075799
- Dun Y, Mysona B, Van Ells T, Amarnath L, Shamsul Ola M, Ganapathy V, Smith SB. Expression of the cystine-glutamate exchanger (x(c) (-)) in retinal ganglion cells and regulation by nitric oxide and oxidative stress. Cell Tissue Res 2006; 324: 189-202. PMID: 16444546
- Gasol E, Jimenez-Vidal M, Chillaron J, Zorzano A, Palacin M. Membrane topology of system xc- light subunit reveals a re-entrant loop with substrate-restricted accessibility.
J Biol Chem 2004; 279: 31228-36. PMID: 15151999
- Gout PW, Buckley AR, Simms CR, Bruchovsky N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the x(c)- cystine transporter: a new action for an old drug. Leukemia. 2001; 15: 1633-40.PMID: 11587223
- Huang Y, Dai Z, Barbacioru C, Sad¨¦e W. Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity and chemoresistance.Cancer Res 2005; 65: 7446-54.
PMID: 16103098
- Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, Masuko T, Shimizu T, Ishikawa T, Kai K, Takahashi E, Imamura Y, Baba Y, Ohmura M, Suematsu M, Baba H, Saya H. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 2011; 19: 387-400. PMID: 21397861
- Jimenez-Vidal M, Gasol E, Zorzano A, Nunes V, Palacin M, Chillaron J. Thiol modification of cysteine 327 in the eighth transmembrane domain of the light subunit xCT of the heteromeric cystine/glutamate antiporter suggests close proximity to the substrate binding site/permeation pathway.
J Biol Chem 2004; 279: 11214-21. PMID: 14722095
- Kaleeba JA, Berger EA. Kaposi's sarcoma-associated herpesvirus fusion-entry receptor: cystine transporter xCT. Science 2006; 311: 1921-4. PMID: 16574866
- Lewerenz J, Maher P. Basal levels of eIF2alpha phosphorylation determine cellular antioxidant status by regulating ATF4 and xCT expression. J Biol Chem 2009; 284: 1106-15. PMID: 19017641
- Liu XX, Li XJ, Zhang B, Liang YJ, Zhou CX, Cao DX, He M, Chen GQ, He JR, Zhao Q.
MicroRNA-26b is underexpressed in human breast cancer and induces cell apoptosis by targeting SLC7A11. FEBS Lett 2011; 585: 1363-7. PMID: 21510944
- Nabeyama A, Kurita A, Asano K, Miyake Y, Yasuda T, Miura I, Nishitai G, Arakawa S, Shimizu S, Wakana S, Yoshida H, Tanaka M. xCT deficiency accelerates chemically induced tumorigenesis. Proc Natl Acad Sci U S A 2010; 107: 6436-41.
PMID: 20308543
- Narang VS, Pauletti GM, Gout PW, Buckley DJ, Buckley AR. Suppression of cystine uptake by sulfasalazine inhibits proliferation of human mammary carcinoma cells.
Anticancer Res 2003; 23: 4571-9.
PMID: 14981898
- Rappocciolo G, Hensler HR, Jais M, Reinhart TA, Pegu A, Jenkins FJ, Rinaldo CR. Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC-SIGN. J Virol 2008; 82: 4793-806.
PMID: 18337571
- Qiang W, Cahill JM, Liu J, Kuang X, Liu N, Scofield VL, Voorhees JR, Reid AJ, Yan M, Lynn WS, Wong PK. Activation of transcription factor Nrf-2 and its downstream targets in response to moloney murine leukemia virus ts1-induced thiol depletion and oxidative stress in astrocytes. J Virol 2004; 78: 11926-38.
PMID: 15479833
- Qiao HX, Hao CJ, Li Y, He X, Chen RS, Cui J, Xu ZH, Li W. JNK activation mediates the apoptosis of xCT-deficient cells. Biochem Biophys Res Commun 2008; 370: 584-8. PMID: 18395005
- Qin Z, Freitas E, Sullivan R, Mohan S, Bacelieri R, Branch D, Romano M, Kearney P, Oates J, Plaisance K, Renne R, Kaleeba J, Parsons C. Upregulation of xCT by KSHV-encoded microRNAs facilitates KSHV dissemination and persistence in an environment of oxidative stress. PLoS Pathog 2010; 6: e1000742. PMID: 20126446
- Sakakura Y, Sato H, Shiiya A, Tamba M, Sagara J, Matsuda M, Okamura N, Makino N, Bannai S.Expression and function of cystine/glutamate transporter in neutrophils. J Leukoc Biol 2007; 81: 974-82. PMID: 17200146
- Sasaki H, Sato H, Kuriyama-Matsumura K, Sato K, Maebara K, Wang H, Tamba M, Itoh K, Yamamoto M, Bannai S. Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J Biol Chem 2002; 277: 44765-71. PMID: 12235164
- Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins.
J Biol Chem 1999; 274:11455-8. PMID: 10206947
- Sato H, Tamba M, Kuriyama-Matsumura K, Okuno S, Bannai S. Molecular cloning and expression of human xCT, the light chain of amino acid transport system xc-.
Antioxid Redox Signal. 2000; 2:665-71. PMID: 11213471
- Sato H, Tamba M, Okuno S, Sato K, Keino-Masu K, Masu M, Bannai S. Distribution of cystine/glutamate exchange transporter, system x(c)-, in the mouse brain.
J Neurosci 2002; 22: 8028-33.PMID: 12223556
- Sato H, Nomura S, Maebara K, Sato K, Tamba M, Bannai S. Transcriptional control of cystine/glutamate transporter gene by amino acid deprivation.
Biochem Biophys Res Commun 2004; 325: 109-16. PMID: 155522208
- Savaskan NE, Heckel A, Hahnen E, Engelhorn T, Doerfler A, Ganslandt O, Nimsky C, Buchfelder M, Ey¨ąpoglu IY. Small interfering RNA-mediated xCT silencing in gliomas inhibits neurodegeneration and alleviates brain edema. Nat Med 2008;14: 629-32.PMID: 18469825
- Veettil MV, Sharma-Walia N, Sadagopan S, Raghu H, Sivakumar R, Naranatt PP, Chandran B. RhoA-GTPase facilitates entry of Kaposi's sarcoma-associated herpesvirus into adherent target cells in a Src-dependent manner.
J Virol 2006; 80: 11432-46. PMID: 17005646
- Veettil MV, Sadagopan S, Sharma-Walia N, Wang FZ, Raghu H, Varga L, Chandran B. Kaposi's sarcoma-associated herpesvirus forms a multimolecular complex of integrins (alphaVbeta5, alphaVbeta3, and alpha3beta1) and CD98-xCT during infection of human dermal microvascular endothelial cells, and CD98-xCT is essential for the postentry stage of infection. J Virol 2008; 82: 12126-44. PMID: 18829766
- Wang H, Tamba M, Kimata M, Sakamoto K, Bannai S, Sato H. Expression of the activity of cystine/glutamate exchange transporter, system x(c)(-), by xCT and rBAT.
Biochem Biophys Res Commun 2003; 305: 611-8. PMID: 12763038
- Zeng Y, Li Y, Chen RS, He X, Yang L, Li W. Overexpression of xCT induces up-regulation of 14-3-3beta in Kaposi's sarcoma. Biosci Rep 2010; 30: 277-83. PMID: 20100173
EDIT HISTORY:
Created by Wei Li, 08/18/2005
Updated by Wei Li, 04/06/2006
Updated by Wei Li, 12/28/2006
Updated by Wei Li, 08/20/2008
Updated by Wei Li, 03/12/2009
Updated by Wei Li, 05/29/2011
(1) Predicted results by ScanProsite:
a) N-glycosylation site: 314 to 317 NFSL.
b) cAMP- and cGMP-dependent protein kinase phosphorylation site: 42 to 45 RKVT; 361 to 364 RKHT.
c) Protein kinase C phosphorylation site: 65 to 67 SPK; 103 to 105 TIK; 187 to 189 SAR; 226 to 228 SGR; 308 to 310 SER; 481 to 483 SEK.
d) Casein kinase II phosphorylation site: 96 to 99 SYAE; 226 to 229 SGRD; 306 to 309 TFSE.
e) N-myristoylation site: 9 sites.
(2) Predicted results of subprograms by PSORT II:
a) N-terminal signal peptide: none
b) 11 tentative TMs, membrane topology: type 3a
c) KDEL ER retention motif in C-terminus: none
d) ER membrane retention signals:
XXRR-like motif in the N-terminus: VRKP; KKXX-like motif in the C-terminus: EEDK
e) VAC possible vacuolar targeting motif: none
f) Actinin-type actin-binding motif: type 1: none; type 2: none
g) Prenylation motif: none
h) memYQRL transport motif from cell surface to Golgi: none
i) Tyrosines in the tail: none
j) Dileucine motif in the tail: none
3D Model
(1)ModBase predicted comparative 3D structure of Q9UPY5 from UCSC Genome Sorter.



From left to right: Front, Top, and Side views of predicted protein.
2D-PAGE
This protein does not exist in the current release of SWISS-2DPAGE.
Computed theoretical MW=55,423Da, pI=9.29 (NP_055146).
FUNCTION
Ontology
(1) Biological process: amino acid transport.
(2) Pheomelanin production.
(3) Oxidative stress.
(4) Belongs to the amino acid-polyamine-organocation (APC) superfamily. L-type amino acid transporter (LAT) (TC 2.A.3.8) family.
Location
Integral membrane protein. Transport system xc- was found in plasma membrane of cultured mammalian cells (Sato, et al (1999)).
Interaction
SLC7A11 (xCT), together with SLC3A2 (4F2hc, or CD98) linked by a disulfide bond at residue C158, encodes the heterodimeric amino acid transport system x(c)(-), which mediates cystine-glutamate exchange and thereby regulates intracellular glutathione levels. System x(c)- activity is important to maintain both intracellular glutathione levels and the redox balance between cystine and cysteine in the extracellular milieu. rBAT can replace 4F2hc in the expression of the activity of system x(c)(-) (Wang, et al).
In mouse NIH3T3 cells, the activity of system x(c)(-) and xCT mRNA is induced not only by deprivation of cystine but also by deprivation of other amino acids via a genomic cis-element termed amino acid response element (AARE) (Sato, et al (2004)). Overexpression of Nrf-2 in astrocytes specifically increases expression of the xCT cystine/glutamate antiporter and other thiol synthesis-related proteins, to promote cell survival (Qiang, et al). Cys(327) is a functionally important residue accessible to the aqueous extracellular environment and is structurally linked to the permeation pathway and/or the substrate binding site (Jimenez-Vidal, et al).
A CD44 variant (CD44v) interacts with xCT and controls the intracellular level of reduced glutathione (GSH). Ablation of CD44 induced loss of xCT from the cell surface and suppressed tumor growth in a transgenic mouse model of gastric cancer. It also induced activation of p38(MAPK), a downstream target of ROS, and expression of the gene for the cell cycle inhibitor p21(CIP1/WAF1)(Ishimoto, et al). xCT has been identified as a receptor mediating KSHV cell fusion (Kaleeba, et al).
miR-26b, which is down-regulated in human breast cancer specimens and cell lines, impairs viability and triggers apoptosis of human breast cancer MCF7 cells. SLC7A11 is identified as a direct target of miR-26b and its expression is remarkably increased in both breast cancer cell lines and clinical samples (Liu, et al).
Pathway
System x(c)(-) is a sodium-independent, high-affinity transporter of anionic amino acids with high specificity for anionic form of cystine and glutamate. Chintala, et al have reported that Slc7a11 regulates the pheomelanin production and cell proliferation. In melanosomes, dopaquinone reacts with cysteine to yield cysteinldopa, which is a precursor of pheomelanin. Cysteine is a component of the tripeptide glutathione. Glutathione metabolism pathway is described in KEGG Pathways.
MUTATION
Allele or SNP
No mutation has been reported in 32 human HPS patients who lack mutations in the seven known HPS genes (Chintala, et al).
SNPs deposited in dbSNP.
Distribution
(none)
Effect
(none)
PHENOTYPE
SLC7A11 is described in OMIM 607933.
Cystine uptake in human cancer cells is largely mediated by system Xc. xCT is relatively highly expressed in lung, colon, and central nervous system cancer cells (Huang, et al). Modulation of xCT activity is possiblely untilized in cancer therapy. Pharmacologic inhibition of system Xc remarkably inhibits the proliferation of several types of cancer cells, including lymphoma, glioma, prostate and breast cancer (Chung, et al; Doxsee, et al; Gout, et al; Narang, et al). This is thought be mediated by the depletion of GSH, which results in an increase of reactive oxygen species (ROS), and ultimately leads to caspase-dependent apoptosis (Doxsee, et al; Sakakura, et al). The apoptosis is likely mediated by c-Jun N-terminal kinase (JNK) activation. The JNK activation triggers both a caspase-dependent (caspases-9 and -3) and an ER stress-mediated (eIF2 and CHOP) pathway to induce apoptosis (Qiao, et al). In the absence of phospho-eIF2alpha, xCT expression is down-regulated that is mediated by nuclear factor ATF4, implicating the role eIF2alpha-ATF4-xCT module in neurodegeneration (Lewerenz, et al).
Disruption of xCT enhanced homotypic cell-cell adhesion and attenuated cell-extracellular matrix adhesion in an esophageal cancer cell line, KYSE150. SASP significantly inhibited both cell invasion of KYSE150 in vitro and its experimental metastasis in nude mice. Caveolin-1 was upregulated and beta-catenin was recruited to the plasma membrane when xCT was deficient, which were followed by the inhibition of beta-catenin transcriptional activity. Upregulation of caveolin-1 and inhibition of tumor cell invasion were mediated by reactive oxygen species-induced p38 MAPK activation (Chen, et al). Savaskan et al show that genetic or pharmacological inhibition of the glutamate transporter Xc system in gliomas leads to abrogated neurodegeneration, attenuated perifocal edema and prolonged survival. xCT deficiency causes unremitting inflammation because of the impaired survival of activated macrophages at the inflammatory site (Nabeyama, et al).
Kaposi's sarcoma-associated herpesvirus (KSHV, human herpesvirus 8) is the causative agent of Kaposi's sarcoma and other lymphoproliferative syndromes often associated with HIV/AIDS. xCT mediates the entry of KSHV which implicates its involvement in the infectious process of this pathogen (Kaleeba, et al; Veettil, et al (2006); Rappocciolo, et al). KSHV interacts with functionally related integrins (alphaVbeta3, alpha3beta1, and alphaVbeta5) and CD98/xCT molecules in a temporal fashion to form a multimolecular complex during the early stages of endothelial cell infection, probably mediating multiple roles in entry, signal transduction, and viral-gene expression (Veettil, et al (2008)). xCT was overexpressed in KS tissues and HHV-8-positive BCBL-1 cells. 14-3-3beta is a downstream effector of xCT in KS to mediate the cell proliferation (Zeng, et al). KSHV-encoded microRNAs (KSHV miRNAs) upregulate xCT expression by macrophages and endothelial cells, largely through miR-K12-11 suppression of BACH-1-a negative regulator of transcription recognizing antioxidant response elements within gene promoters (Qin, et al).
REFERENCE
- Chen RS, Song YM, Zhou ZY, Tong T, Li Y, Fu M, Guo XL, Dong LJ, He X, Qiao HX, Zhan QM, Li W. Disruption of xCT inhibits cancer cell metastasis via the caveolin-1/beta-catenin pathway. Oncogene 2009; 28: 599-609. PMID: 19015640
- Chintala S, Li W, Lamoreux ML, Ito S, Wakamatsu K, Sviderskaya EV, Bennett DC, Park YM, Gahl WA, Huizing M, Spritz RA, Ben S, Novak EK, Tan J, Swank RT.
Slc7a11 gene controls production of pheomelanin pigment and proliferation of cultured cells. Proc Natl Acad Sci U S A 2005; 102: 10964-9. PMID: 16037214
- Chung WJ, Lyons SA, Nelson GM, Hamza H, Gladson CL, Gillespie GY, Sontheimer H. Inhibition of cystine uptake disrupts the growth of primary brain tumors.
J Neurosci 2005; 25: 7101-10.
PMID: 16079392
- Doxsee DW, Gout PW, Kurita T, Lo M, Buckley AR, Wang Y, Xue H, Karp CM, Cutz JC, Cunha GR, Wang YZ. Sulfasalazine-induced cystine starvation: potential use for prostate cancer therapy. Prostate 2007; 67: 162-71.
PMID: 17075799
- Dun Y, Mysona B, Van Ells T, Amarnath L, Shamsul Ola M, Ganapathy V, Smith SB. Expression of the cystine-glutamate exchanger (x(c) (-)) in retinal ganglion cells and regulation by nitric oxide and oxidative stress. Cell Tissue Res 2006; 324: 189-202. PMID: 16444546
- Gasol E, Jimenez-Vidal M, Chillaron J, Zorzano A, Palacin M. Membrane topology of system xc- light subunit reveals a re-entrant loop with substrate-restricted accessibility.
J Biol Chem 2004; 279: 31228-36. PMID: 15151999
- Gout PW, Buckley AR, Simms CR, Bruchovsky N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the x(c)- cystine transporter: a new action for an old drug. Leukemia. 2001; 15: 1633-40.PMID: 11587223
- Huang Y, Dai Z, Barbacioru C, Sad¨¦e W. Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity and chemoresistance.Cancer Res 2005; 65: 7446-54.
PMID: 16103098
- Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, Masuko T, Shimizu T, Ishikawa T, Kai K, Takahashi E, Imamura Y, Baba Y, Ohmura M, Suematsu M, Baba H, Saya H. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 2011; 19: 387-400. PMID: 21397861
- Jimenez-Vidal M, Gasol E, Zorzano A, Nunes V, Palacin M, Chillaron J. Thiol modification of cysteine 327 in the eighth transmembrane domain of the light subunit xCT of the heteromeric cystine/glutamate antiporter suggests close proximity to the substrate binding site/permeation pathway.
J Biol Chem 2004; 279: 11214-21. PMID: 14722095
- Kaleeba JA, Berger EA. Kaposi's sarcoma-associated herpesvirus fusion-entry receptor: cystine transporter xCT. Science 2006; 311: 1921-4. PMID: 16574866
- Lewerenz J, Maher P. Basal levels of eIF2alpha phosphorylation determine cellular antioxidant status by regulating ATF4 and xCT expression. J Biol Chem 2009; 284: 1106-15. PMID: 19017641
- Liu XX, Li XJ, Zhang B, Liang YJ, Zhou CX, Cao DX, He M, Chen GQ, He JR, Zhao Q.
MicroRNA-26b is underexpressed in human breast cancer and induces cell apoptosis by targeting SLC7A11. FEBS Lett 2011; 585: 1363-7. PMID: 21510944
- Nabeyama A, Kurita A, Asano K, Miyake Y, Yasuda T, Miura I, Nishitai G, Arakawa S, Shimizu S, Wakana S, Yoshida H, Tanaka M. xCT deficiency accelerates chemically induced tumorigenesis. Proc Natl Acad Sci U S A 2010; 107: 6436-41.
PMID: 20308543
- Narang VS, Pauletti GM, Gout PW, Buckley DJ, Buckley AR. Suppression of cystine uptake by sulfasalazine inhibits proliferation of human mammary carcinoma cells.
Anticancer Res 2003; 23: 4571-9.
PMID: 14981898
- Rappocciolo G, Hensler HR, Jais M, Reinhart TA, Pegu A, Jenkins FJ, Rinaldo CR. Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC-SIGN. J Virol 2008; 82: 4793-806.
PMID: 18337571
- Qiang W, Cahill JM, Liu J, Kuang X, Liu N, Scofield VL, Voorhees JR, Reid AJ, Yan M, Lynn WS, Wong PK. Activation of transcription factor Nrf-2 and its downstream targets in response to moloney murine leukemia virus ts1-induced thiol depletion and oxidative stress in astrocytes. J Virol 2004; 78: 11926-38.
PMID: 15479833
- Qiao HX, Hao CJ, Li Y, He X, Chen RS, Cui J, Xu ZH, Li W. JNK activation mediates the apoptosis of xCT-deficient cells. Biochem Biophys Res Commun 2008; 370: 584-8. PMID: 18395005
- Qin Z, Freitas E, Sullivan R, Mohan S, Bacelieri R, Branch D, Romano M, Kearney P, Oates J, Plaisance K, Renne R, Kaleeba J, Parsons C. Upregulation of xCT by KSHV-encoded microRNAs facilitates KSHV dissemination and persistence in an environment of oxidative stress. PLoS Pathog 2010; 6: e1000742. PMID: 20126446
- Sakakura Y, Sato H, Shiiya A, Tamba M, Sagara J, Matsuda M, Okamura N, Makino N, Bannai S.Expression and function of cystine/glutamate transporter in neutrophils. J Leukoc Biol 2007; 81: 974-82. PMID: 17200146
- Sasaki H, Sato H, Kuriyama-Matsumura K, Sato K, Maebara K, Wang H, Tamba M, Itoh K, Yamamoto M, Bannai S. Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J Biol Chem 2002; 277: 44765-71. PMID: 12235164
- Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins.
J Biol Chem 1999; 274:11455-8. PMID: 10206947
- Sato H, Tamba M, Kuriyama-Matsumura K, Okuno S, Bannai S. Molecular cloning and expression of human xCT, the light chain of amino acid transport system xc-.
Antioxid Redox Signal. 2000; 2:665-71. PMID: 11213471
- Sato H, Tamba M, Okuno S, Sato K, Keino-Masu K, Masu M, Bannai S. Distribution of cystine/glutamate exchange transporter, system x(c)-, in the mouse brain.
J Neurosci 2002; 22: 8028-33.PMID: 12223556
- Sato H, Nomura S, Maebara K, Sato K, Tamba M, Bannai S. Transcriptional control of cystine/glutamate transporter gene by amino acid deprivation.
Biochem Biophys Res Commun 2004; 325: 109-16. PMID: 155522208
- Savaskan NE, Heckel A, Hahnen E, Engelhorn T, Doerfler A, Ganslandt O, Nimsky C, Buchfelder M, Ey¨ąpoglu IY. Small interfering RNA-mediated xCT silencing in gliomas inhibits neurodegeneration and alleviates brain edema. Nat Med 2008;14: 629-32.PMID: 18469825
- Veettil MV, Sharma-Walia N, Sadagopan S, Raghu H, Sivakumar R, Naranatt PP, Chandran B. RhoA-GTPase facilitates entry of Kaposi's sarcoma-associated herpesvirus into adherent target cells in a Src-dependent manner.
J Virol 2006; 80: 11432-46. PMID: 17005646
- Veettil MV, Sadagopan S, Sharma-Walia N, Wang FZ, Raghu H, Varga L, Chandran B. Kaposi's sarcoma-associated herpesvirus forms a multimolecular complex of integrins (alphaVbeta5, alphaVbeta3, and alpha3beta1) and CD98-xCT during infection of human dermal microvascular endothelial cells, and CD98-xCT is essential for the postentry stage of infection. J Virol 2008; 82: 12126-44. PMID: 18829766
- Wang H, Tamba M, Kimata M, Sakamoto K, Bannai S, Sato H. Expression of the activity of cystine/glutamate exchange transporter, system x(c)(-), by xCT and rBAT.
Biochem Biophys Res Commun 2003; 305: 611-8. PMID: 12763038
- Zeng Y, Li Y, Chen RS, He X, Yang L, Li W. Overexpression of xCT induces up-regulation of 14-3-3beta in Kaposi's sarcoma. Biosci Rep 2010; 30: 277-83. PMID: 20100173
EDIT HISTORY:
Created by Wei Li, 08/18/2005
Updated by Wei Li, 04/06/2006
Updated by Wei Li, 12/28/2006
Updated by Wei Li, 08/20/2008
Updated by Wei Li, 03/12/2009
Updated by Wei Li, 05/29/2011
(1)ModBase predicted comparative 3D structure of Q9UPY5 from UCSC Genome Sorter.



From left to right: Front, Top, and Side views of predicted protein.
2D-PAGE
This protein does not exist in the current release of SWISS-2DPAGE.
Computed theoretical MW=55,423Da, pI=9.29 (NP_055146).
FUNCTION
Ontology
(1) Biological process: amino acid transport.
(2) Pheomelanin production.
(3) Oxidative stress.
(4) Belongs to the amino acid-polyamine-organocation (APC) superfamily. L-type amino acid transporter (LAT) (TC 2.A.3.8) family.
Location
Integral membrane protein. Transport system xc- was found in plasma membrane of cultured mammalian cells (Sato, et al (1999)).
Interaction
SLC7A11 (xCT), together with SLC3A2 (4F2hc, or CD98) linked by a disulfide bond at residue C158, encodes the heterodimeric amino acid transport system x(c)(-), which mediates cystine-glutamate exchange and thereby regulates intracellular glutathione levels. System x(c)- activity is important to maintain both intracellular glutathione levels and the redox balance between cystine and cysteine in the extracellular milieu. rBAT can replace 4F2hc in the expression of the activity of system x(c)(-) (Wang, et al).
In mouse NIH3T3 cells, the activity of system x(c)(-) and xCT mRNA is induced not only by deprivation of cystine but also by deprivation of other amino acids via a genomic cis-element termed amino acid response element (AARE) (Sato, et al (2004)). Overexpression of Nrf-2 in astrocytes specifically increases expression of the xCT cystine/glutamate antiporter and other thiol synthesis-related proteins, to promote cell survival (Qiang, et al). Cys(327) is a functionally important residue accessible to the aqueous extracellular environment and is structurally linked to the permeation pathway and/or the substrate binding site (Jimenez-Vidal, et al).
A CD44 variant (CD44v) interacts with xCT and controls the intracellular level of reduced glutathione (GSH). Ablation of CD44 induced loss of xCT from the cell surface and suppressed tumor growth in a transgenic mouse model of gastric cancer. It also induced activation of p38(MAPK), a downstream target of ROS, and expression of the gene for the cell cycle inhibitor p21(CIP1/WAF1)(Ishimoto, et al). xCT has been identified as a receptor mediating KSHV cell fusion (Kaleeba, et al).
miR-26b, which is down-regulated in human breast cancer specimens and cell lines, impairs viability and triggers apoptosis of human breast cancer MCF7 cells. SLC7A11 is identified as a direct target of miR-26b and its expression is remarkably increased in both breast cancer cell lines and clinical samples (Liu, et al).
Pathway
System x(c)(-) is a sodium-independent, high-affinity transporter of anionic amino acids with high specificity for anionic form of cystine and glutamate. Chintala, et al have reported that Slc7a11 regulates the pheomelanin production and cell proliferation. In melanosomes, dopaquinone reacts with cysteine to yield cysteinldopa, which is a precursor of pheomelanin. Cysteine is a component of the tripeptide glutathione. Glutathione metabolism pathway is described in KEGG Pathways.
MUTATION
Allele or SNP
No mutation has been reported in 32 human HPS patients who lack mutations in the seven known HPS genes (Chintala, et al).
SNPs deposited in dbSNP.
Distribution
(none)
Effect
(none)
PHENOTYPE
SLC7A11 is described in OMIM 607933.
Cystine uptake in human cancer cells is largely mediated by system Xc. xCT is relatively highly expressed in lung, colon, and central nervous system cancer cells (Huang, et al). Modulation of xCT activity is possiblely untilized in cancer therapy. Pharmacologic inhibition of system Xc remarkably inhibits the proliferation of several types of cancer cells, including lymphoma, glioma, prostate and breast cancer (Chung, et al; Doxsee, et al; Gout, et al; Narang, et al). This is thought be mediated by the depletion of GSH, which results in an increase of reactive oxygen species (ROS), and ultimately leads to caspase-dependent apoptosis (Doxsee, et al; Sakakura, et al). The apoptosis is likely mediated by c-Jun N-terminal kinase (JNK) activation. The JNK activation triggers both a caspase-dependent (caspases-9 and -3) and an ER stress-mediated (eIF2 and CHOP) pathway to induce apoptosis (Qiao, et al). In the absence of phospho-eIF2alpha, xCT expression is down-regulated that is mediated by nuclear factor ATF4, implicating the role eIF2alpha-ATF4-xCT module in neurodegeneration (Lewerenz, et al).
Disruption of xCT enhanced homotypic cell-cell adhesion and attenuated cell-extracellular matrix adhesion in an esophageal cancer cell line, KYSE150. SASP significantly inhibited both cell invasion of KYSE150 in vitro and its experimental metastasis in nude mice. Caveolin-1 was upregulated and beta-catenin was recruited to the plasma membrane when xCT was deficient, which were followed by the inhibition of beta-catenin transcriptional activity. Upregulation of caveolin-1 and inhibition of tumor cell invasion were mediated by reactive oxygen species-induced p38 MAPK activation (Chen, et al). Savaskan et al show that genetic or pharmacological inhibition of the glutamate transporter Xc system in gliomas leads to abrogated neurodegeneration, attenuated perifocal edema and prolonged survival. xCT deficiency causes unremitting inflammation because of the impaired survival of activated macrophages at the inflammatory site (Nabeyama, et al).
Kaposi's sarcoma-associated herpesvirus (KSHV, human herpesvirus 8) is the causative agent of Kaposi's sarcoma and other lymphoproliferative syndromes often associated with HIV/AIDS. xCT mediates the entry of KSHV which implicates its involvement in the infectious process of this pathogen (Kaleeba, et al; Veettil, et al (2006); Rappocciolo, et al). KSHV interacts with functionally related integrins (alphaVbeta3, alpha3beta1, and alphaVbeta5) and CD98/xCT molecules in a temporal fashion to form a multimolecular complex during the early stages of endothelial cell infection, probably mediating multiple roles in entry, signal transduction, and viral-gene expression (Veettil, et al (2008)). xCT was overexpressed in KS tissues and HHV-8-positive BCBL-1 cells. 14-3-3beta is a downstream effector of xCT in KS to mediate the cell proliferation (Zeng, et al). KSHV-encoded microRNAs (KSHV miRNAs) upregulate xCT expression by macrophages and endothelial cells, largely through miR-K12-11 suppression of BACH-1-a negative regulator of transcription recognizing antioxidant response elements within gene promoters (Qin, et al).
REFERENCE
- Chen RS, Song YM, Zhou ZY, Tong T, Li Y, Fu M, Guo XL, Dong LJ, He X, Qiao HX, Zhan QM, Li W. Disruption of xCT inhibits cancer cell metastasis via the caveolin-1/beta-catenin pathway. Oncogene 2009; 28: 599-609. PMID: 19015640
- Chintala S, Li W, Lamoreux ML, Ito S, Wakamatsu K, Sviderskaya EV, Bennett DC, Park YM, Gahl WA, Huizing M, Spritz RA, Ben S, Novak EK, Tan J, Swank RT.
Slc7a11 gene controls production of pheomelanin pigment and proliferation of cultured cells. Proc Natl Acad Sci U S A 2005; 102: 10964-9. PMID: 16037214
- Chung WJ, Lyons SA, Nelson GM, Hamza H, Gladson CL, Gillespie GY, Sontheimer H. Inhibition of cystine uptake disrupts the growth of primary brain tumors.
J Neurosci 2005; 25: 7101-10.
PMID: 16079392
- Doxsee DW, Gout PW, Kurita T, Lo M, Buckley AR, Wang Y, Xue H, Karp CM, Cutz JC, Cunha GR, Wang YZ. Sulfasalazine-induced cystine starvation: potential use for prostate cancer therapy. Prostate 2007; 67: 162-71.
PMID: 17075799
- Dun Y, Mysona B, Van Ells T, Amarnath L, Shamsul Ola M, Ganapathy V, Smith SB. Expression of the cystine-glutamate exchanger (x(c) (-)) in retinal ganglion cells and regulation by nitric oxide and oxidative stress. Cell Tissue Res 2006; 324: 189-202. PMID: 16444546
- Gasol E, Jimenez-Vidal M, Chillaron J, Zorzano A, Palacin M. Membrane topology of system xc- light subunit reveals a re-entrant loop with substrate-restricted accessibility.
J Biol Chem 2004; 279: 31228-36. PMID: 15151999
- Gout PW, Buckley AR, Simms CR, Bruchovsky N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the x(c)- cystine transporter: a new action for an old drug. Leukemia. 2001; 15: 1633-40.PMID: 11587223
- Huang Y, Dai Z, Barbacioru C, Sad¨¦e W. Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity and chemoresistance.Cancer Res 2005; 65: 7446-54.
PMID: 16103098
- Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, Masuko T, Shimizu T, Ishikawa T, Kai K, Takahashi E, Imamura Y, Baba Y, Ohmura M, Suematsu M, Baba H, Saya H. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 2011; 19: 387-400. PMID: 21397861
- Jimenez-Vidal M, Gasol E, Zorzano A, Nunes V, Palacin M, Chillaron J. Thiol modification of cysteine 327 in the eighth transmembrane domain of the light subunit xCT of the heteromeric cystine/glutamate antiporter suggests close proximity to the substrate binding site/permeation pathway.
J Biol Chem 2004; 279: 11214-21. PMID: 14722095
- Kaleeba JA, Berger EA. Kaposi's sarcoma-associated herpesvirus fusion-entry receptor: cystine transporter xCT. Science 2006; 311: 1921-4. PMID: 16574866
- Lewerenz J, Maher P. Basal levels of eIF2alpha phosphorylation determine cellular antioxidant status by regulating ATF4 and xCT expression. J Biol Chem 2009; 284: 1106-15. PMID: 19017641
- Liu XX, Li XJ, Zhang B, Liang YJ, Zhou CX, Cao DX, He M, Chen GQ, He JR, Zhao Q.
MicroRNA-26b is underexpressed in human breast cancer and induces cell apoptosis by targeting SLC7A11. FEBS Lett 2011; 585: 1363-7. PMID: 21510944
- Nabeyama A, Kurita A, Asano K, Miyake Y, Yasuda T, Miura I, Nishitai G, Arakawa S, Shimizu S, Wakana S, Yoshida H, Tanaka M. xCT deficiency accelerates chemically induced tumorigenesis. Proc Natl Acad Sci U S A 2010; 107: 6436-41.
PMID: 20308543
- Narang VS, Pauletti GM, Gout PW, Buckley DJ, Buckley AR. Suppression of cystine uptake by sulfasalazine inhibits proliferation of human mammary carcinoma cells.
Anticancer Res 2003; 23: 4571-9.
PMID: 14981898
- Rappocciolo G, Hensler HR, Jais M, Reinhart TA, Pegu A, Jenkins FJ, Rinaldo CR. Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC-SIGN. J Virol 2008; 82: 4793-806.
PMID: 18337571
- Qiang W, Cahill JM, Liu J, Kuang X, Liu N, Scofield VL, Voorhees JR, Reid AJ, Yan M, Lynn WS, Wong PK. Activation of transcription factor Nrf-2 and its downstream targets in response to moloney murine leukemia virus ts1-induced thiol depletion and oxidative stress in astrocytes. J Virol 2004; 78: 11926-38.
PMID: 15479833
- Qiao HX, Hao CJ, Li Y, He X, Chen RS, Cui J, Xu ZH, Li W. JNK activation mediates the apoptosis of xCT-deficient cells. Biochem Biophys Res Commun 2008; 370: 584-8. PMID: 18395005
- Qin Z, Freitas E, Sullivan R, Mohan S, Bacelieri R, Branch D, Romano M, Kearney P, Oates J, Plaisance K, Renne R, Kaleeba J, Parsons C. Upregulation of xCT by KSHV-encoded microRNAs facilitates KSHV dissemination and persistence in an environment of oxidative stress. PLoS Pathog 2010; 6: e1000742. PMID: 20126446
- Sakakura Y, Sato H, Shiiya A, Tamba M, Sagara J, Matsuda M, Okamura N, Makino N, Bannai S.Expression and function of cystine/glutamate transporter in neutrophils. J Leukoc Biol 2007; 81: 974-82. PMID: 17200146
- Sasaki H, Sato H, Kuriyama-Matsumura K, Sato K, Maebara K, Wang H, Tamba M, Itoh K, Yamamoto M, Bannai S. Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J Biol Chem 2002; 277: 44765-71. PMID: 12235164
- Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins.
J Biol Chem 1999; 274:11455-8. PMID: 10206947
- Sato H, Tamba M, Kuriyama-Matsumura K, Okuno S, Bannai S. Molecular cloning and expression of human xCT, the light chain of amino acid transport system xc-.
Antioxid Redox Signal. 2000; 2:665-71. PMID: 11213471
- Sato H, Tamba M, Okuno S, Sato K, Keino-Masu K, Masu M, Bannai S. Distribution of cystine/glutamate exchange transporter, system x(c)-, in the mouse brain.
J Neurosci 2002; 22: 8028-33.PMID: 12223556
- Sato H, Nomura S, Maebara K, Sato K, Tamba M, Bannai S. Transcriptional control of cystine/glutamate transporter gene by amino acid deprivation.
Biochem Biophys Res Commun 2004; 325: 109-16. PMID: 155522208
- Savaskan NE, Heckel A, Hahnen E, Engelhorn T, Doerfler A, Ganslandt O, Nimsky C, Buchfelder M, Ey¨ąpoglu IY. Small interfering RNA-mediated xCT silencing in gliomas inhibits neurodegeneration and alleviates brain edema. Nat Med 2008;14: 629-32.PMID: 18469825
- Veettil MV, Sharma-Walia N, Sadagopan S, Raghu H, Sivakumar R, Naranatt PP, Chandran B. RhoA-GTPase facilitates entry of Kaposi's sarcoma-associated herpesvirus into adherent target cells in a Src-dependent manner.
J Virol 2006; 80: 11432-46. PMID: 17005646
- Veettil MV, Sadagopan S, Sharma-Walia N, Wang FZ, Raghu H, Varga L, Chandran B. Kaposi's sarcoma-associated herpesvirus forms a multimolecular complex of integrins (alphaVbeta5, alphaVbeta3, and alpha3beta1) and CD98-xCT during infection of human dermal microvascular endothelial cells, and CD98-xCT is essential for the postentry stage of infection. J Virol 2008; 82: 12126-44. PMID: 18829766
- Wang H, Tamba M, Kimata M, Sakamoto K, Bannai S, Sato H. Expression of the activity of cystine/glutamate exchange transporter, system x(c)(-), by xCT and rBAT.
Biochem Biophys Res Commun 2003; 305: 611-8. PMID: 12763038
- Zeng Y, Li Y, Chen RS, He X, Yang L, Li W. Overexpression of xCT induces up-regulation of 14-3-3beta in Kaposi's sarcoma. Biosci Rep 2010; 30: 277-83. PMID: 20100173
EDIT HISTORY:
Created by Wei Li, 08/18/2005
Updated by Wei Li, 04/06/2006
Updated by Wei Li, 12/28/2006
Updated by Wei Li, 08/20/2008
Updated by Wei Li, 03/12/2009
Updated by Wei Li, 05/29/2011
This protein does not exist in the current release of SWISS-2DPAGE.
Computed theoretical MW=55,423Da, pI=9.29 (NP_055146).
FUNCTION
Ontology
(1) Biological process: amino acid transport.
(2) Pheomelanin production.
(3) Oxidative stress.
(4) Belongs to the amino acid-polyamine-organocation (APC) superfamily. L-type amino acid transporter (LAT) (TC 2.A.3.8) family.
Location
Integral membrane protein. Transport system xc- was found in plasma membrane of cultured mammalian cells (Sato, et al (1999)).
Interaction
SLC7A11 (xCT), together with SLC3A2 (4F2hc, or CD98) linked by a disulfide bond at residue C158, encodes the heterodimeric amino acid transport system x(c)(-), which mediates cystine-glutamate exchange and thereby regulates intracellular glutathione levels. System x(c)- activity is important to maintain both intracellular glutathione levels and the redox balance between cystine and cysteine in the extracellular milieu. rBAT can replace 4F2hc in the expression of the activity of system x(c)(-) (Wang, et al).
In mouse NIH3T3 cells, the activity of system x(c)(-) and xCT mRNA is induced not only by deprivation of cystine but also by deprivation of other amino acids via a genomic cis-element termed amino acid response element (AARE) (Sato, et al (2004)). Overexpression of Nrf-2 in astrocytes specifically increases expression of the xCT cystine/glutamate antiporter and other thiol synthesis-related proteins, to promote cell survival (Qiang, et al). Cys(327) is a functionally important residue accessible to the aqueous extracellular environment and is structurally linked to the permeation pathway and/or the substrate binding site (Jimenez-Vidal, et al).
A CD44 variant (CD44v) interacts with xCT and controls the intracellular level of reduced glutathione (GSH). Ablation of CD44 induced loss of xCT from the cell surface and suppressed tumor growth in a transgenic mouse model of gastric cancer. It also induced activation of p38(MAPK), a downstream target of ROS, and expression of the gene for the cell cycle inhibitor p21(CIP1/WAF1)(Ishimoto, et al). xCT has been identified as a receptor mediating KSHV cell fusion (Kaleeba, et al).
miR-26b, which is down-regulated in human breast cancer specimens and cell lines, impairs viability and triggers apoptosis of human breast cancer MCF7 cells. SLC7A11 is identified as a direct target of miR-26b and its expression is remarkably increased in both breast cancer cell lines and clinical samples (Liu, et al).
Pathway
System x(c)(-) is a sodium-independent, high-affinity transporter of anionic amino acids with high specificity for anionic form of cystine and glutamate. Chintala, et al have reported that Slc7a11 regulates the pheomelanin production and cell proliferation. In melanosomes, dopaquinone reacts with cysteine to yield cysteinldopa, which is a precursor of pheomelanin. Cysteine is a component of the tripeptide glutathione. Glutathione metabolism pathway is described in KEGG Pathways.
MUTATION
Allele or SNP
No mutation has been reported in 32 human HPS patients who lack mutations in the seven known HPS genes (Chintala, et al).
SNPs deposited in dbSNP.
Distribution
(none)
Effect
(none)
PHENOTYPE
SLC7A11 is described in OMIM 607933.
Cystine uptake in human cancer cells is largely mediated by system Xc. xCT is relatively highly expressed in lung, colon, and central nervous system cancer cells (Huang, et al). Modulation of xCT activity is possiblely untilized in cancer therapy. Pharmacologic inhibition of system Xc remarkably inhibits the proliferation of several types of cancer cells, including lymphoma, glioma, prostate and breast cancer (Chung, et al; Doxsee, et al; Gout, et al; Narang, et al). This is thought be mediated by the depletion of GSH, which results in an increase of reactive oxygen species (ROS), and ultimately leads to caspase-dependent apoptosis (Doxsee, et al; Sakakura, et al). The apoptosis is likely mediated by c-Jun N-terminal kinase (JNK) activation. The JNK activation triggers both a caspase-dependent (caspases-9 and -3) and an ER stress-mediated (eIF2 and CHOP) pathway to induce apoptosis (Qiao, et al). In the absence of phospho-eIF2alpha, xCT expression is down-regulated that is mediated by nuclear factor ATF4, implicating the role eIF2alpha-ATF4-xCT module in neurodegeneration (Lewerenz, et al).
Disruption of xCT enhanced homotypic cell-cell adhesion and attenuated cell-extracellular matrix adhesion in an esophageal cancer cell line, KYSE150. SASP significantly inhibited both cell invasion of KYSE150 in vitro and its experimental metastasis in nude mice. Caveolin-1 was upregulated and beta-catenin was recruited to the plasma membrane when xCT was deficient, which were followed by the inhibition of beta-catenin transcriptional activity. Upregulation of caveolin-1 and inhibition of tumor cell invasion were mediated by reactive oxygen species-induced p38 MAPK activation (Chen, et al). Savaskan et al show that genetic or pharmacological inhibition of the glutamate transporter Xc system in gliomas leads to abrogated neurodegeneration, attenuated perifocal edema and prolonged survival. xCT deficiency causes unremitting inflammation because of the impaired survival of activated macrophages at the inflammatory site (Nabeyama, et al).
Kaposi's sarcoma-associated herpesvirus (KSHV, human herpesvirus 8) is the causative agent of Kaposi's sarcoma and other lymphoproliferative syndromes often associated with HIV/AIDS. xCT mediates the entry of KSHV which implicates its involvement in the infectious process of this pathogen (Kaleeba, et al; Veettil, et al (2006); Rappocciolo, et al). KSHV interacts with functionally related integrins (alphaVbeta3, alpha3beta1, and alphaVbeta5) and CD98/xCT molecules in a temporal fashion to form a multimolecular complex during the early stages of endothelial cell infection, probably mediating multiple roles in entry, signal transduction, and viral-gene expression (Veettil, et al (2008)). xCT was overexpressed in KS tissues and HHV-8-positive BCBL-1 cells. 14-3-3beta is a downstream effector of xCT in KS to mediate the cell proliferation (Zeng, et al). KSHV-encoded microRNAs (KSHV miRNAs) upregulate xCT expression by macrophages and endothelial cells, largely through miR-K12-11 suppression of BACH-1-a negative regulator of transcription recognizing antioxidant response elements within gene promoters (Qin, et al).
REFERENCE
- Chen RS, Song YM, Zhou ZY, Tong T, Li Y, Fu M, Guo XL, Dong LJ, He X, Qiao HX, Zhan QM, Li W. Disruption of xCT inhibits cancer cell metastasis via the caveolin-1/beta-catenin pathway. Oncogene 2009; 28: 599-609. PMID: 19015640
- Chintala S, Li W, Lamoreux ML, Ito S, Wakamatsu K, Sviderskaya EV, Bennett DC, Park YM, Gahl WA, Huizing M, Spritz RA, Ben S, Novak EK, Tan J, Swank RT.
Slc7a11 gene controls production of pheomelanin pigment and proliferation of cultured cells. Proc Natl Acad Sci U S A 2005; 102: 10964-9. PMID: 16037214
- Chung WJ, Lyons SA, Nelson GM, Hamza H, Gladson CL, Gillespie GY, Sontheimer H. Inhibition of cystine uptake disrupts the growth of primary brain tumors.
J Neurosci 2005; 25: 7101-10.
PMID: 16079392
- Doxsee DW, Gout PW, Kurita T, Lo M, Buckley AR, Wang Y, Xue H, Karp CM, Cutz JC, Cunha GR, Wang YZ. Sulfasalazine-induced cystine starvation: potential use for prostate cancer therapy. Prostate 2007; 67: 162-71.
PMID: 17075799
- Dun Y, Mysona B, Van Ells T, Amarnath L, Shamsul Ola M, Ganapathy V, Smith SB. Expression of the cystine-glutamate exchanger (x(c) (-)) in retinal ganglion cells and regulation by nitric oxide and oxidative stress. Cell Tissue Res 2006; 324: 189-202. PMID: 16444546
- Gasol E, Jimenez-Vidal M, Chillaron J, Zorzano A, Palacin M. Membrane topology of system xc- light subunit reveals a re-entrant loop with substrate-restricted accessibility.
J Biol Chem 2004; 279: 31228-36. PMID: 15151999
- Gout PW, Buckley AR, Simms CR, Bruchovsky N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the x(c)- cystine transporter: a new action for an old drug. Leukemia. 2001; 15: 1633-40.PMID: 11587223
- Huang Y, Dai Z, Barbacioru C, Sad¨¦e W. Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity and chemoresistance.Cancer Res 2005; 65: 7446-54.
PMID: 16103098
- Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, Masuko T, Shimizu T, Ishikawa T, Kai K, Takahashi E, Imamura Y, Baba Y, Ohmura M, Suematsu M, Baba H, Saya H. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 2011; 19: 387-400. PMID: 21397861
- Jimenez-Vidal M, Gasol E, Zorzano A, Nunes V, Palacin M, Chillaron J. Thiol modification of cysteine 327 in the eighth transmembrane domain of the light subunit xCT of the heteromeric cystine/glutamate antiporter suggests close proximity to the substrate binding site/permeation pathway.
J Biol Chem 2004; 279: 11214-21. PMID: 14722095
- Kaleeba JA, Berger EA. Kaposi's sarcoma-associated herpesvirus fusion-entry receptor: cystine transporter xCT. Science 2006; 311: 1921-4. PMID: 16574866
- Lewerenz J, Maher P. Basal levels of eIF2alpha phosphorylation determine cellular antioxidant status by regulating ATF4 and xCT expression. J Biol Chem 2009; 284: 1106-15. PMID: 19017641
- Liu XX, Li XJ, Zhang B, Liang YJ, Zhou CX, Cao DX, He M, Chen GQ, He JR, Zhao Q.
MicroRNA-26b is underexpressed in human breast cancer and induces cell apoptosis by targeting SLC7A11. FEBS Lett 2011; 585: 1363-7. PMID: 21510944
- Nabeyama A, Kurita A, Asano K, Miyake Y, Yasuda T, Miura I, Nishitai G, Arakawa S, Shimizu S, Wakana S, Yoshida H, Tanaka M. xCT deficiency accelerates chemically induced tumorigenesis. Proc Natl Acad Sci U S A 2010; 107: 6436-41.
PMID: 20308543
- Narang VS, Pauletti GM, Gout PW, Buckley DJ, Buckley AR. Suppression of cystine uptake by sulfasalazine inhibits proliferation of human mammary carcinoma cells.
Anticancer Res 2003; 23: 4571-9.
PMID: 14981898
- Rappocciolo G, Hensler HR, Jais M, Reinhart TA, Pegu A, Jenkins FJ, Rinaldo CR. Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC-SIGN. J Virol 2008; 82: 4793-806.
PMID: 18337571
- Qiang W, Cahill JM, Liu J, Kuang X, Liu N, Scofield VL, Voorhees JR, Reid AJ, Yan M, Lynn WS, Wong PK. Activation of transcription factor Nrf-2 and its downstream targets in response to moloney murine leukemia virus ts1-induced thiol depletion and oxidative stress in astrocytes. J Virol 2004; 78: 11926-38.
PMID: 15479833
- Qiao HX, Hao CJ, Li Y, He X, Chen RS, Cui J, Xu ZH, Li W. JNK activation mediates the apoptosis of xCT-deficient cells. Biochem Biophys Res Commun 2008; 370: 584-8. PMID: 18395005
- Qin Z, Freitas E, Sullivan R, Mohan S, Bacelieri R, Branch D, Romano M, Kearney P, Oates J, Plaisance K, Renne R, Kaleeba J, Parsons C. Upregulation of xCT by KSHV-encoded microRNAs facilitates KSHV dissemination and persistence in an environment of oxidative stress. PLoS Pathog 2010; 6: e1000742. PMID: 20126446
- Sakakura Y, Sato H, Shiiya A, Tamba M, Sagara J, Matsuda M, Okamura N, Makino N, Bannai S.Expression and function of cystine/glutamate transporter in neutrophils. J Leukoc Biol 2007; 81: 974-82. PMID: 17200146
- Sasaki H, Sato H, Kuriyama-Matsumura K, Sato K, Maebara K, Wang H, Tamba M, Itoh K, Yamamoto M, Bannai S. Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J Biol Chem 2002; 277: 44765-71. PMID: 12235164
- Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins.
J Biol Chem 1999; 274:11455-8. PMID: 10206947
- Sato H, Tamba M, Kuriyama-Matsumura K, Okuno S, Bannai S. Molecular cloning and expression of human xCT, the light chain of amino acid transport system xc-.
Antioxid Redox Signal. 2000; 2:665-71. PMID: 11213471
- Sato H, Tamba M, Okuno S, Sato K, Keino-Masu K, Masu M, Bannai S. Distribution of cystine/glutamate exchange transporter, system x(c)-, in the mouse brain.
J Neurosci 2002; 22: 8028-33.PMID: 12223556
- Sato H, Nomura S, Maebara K, Sato K, Tamba M, Bannai S. Transcriptional control of cystine/glutamate transporter gene by amino acid deprivation.
Biochem Biophys Res Commun 2004; 325: 109-16. PMID: 155522208
- Savaskan NE, Heckel A, Hahnen E, Engelhorn T, Doerfler A, Ganslandt O, Nimsky C, Buchfelder M, Ey¨ąpoglu IY. Small interfering RNA-mediated xCT silencing in gliomas inhibits neurodegeneration and alleviates brain edema. Nat Med 2008;14: 629-32.PMID: 18469825
- Veettil MV, Sharma-Walia N, Sadagopan S, Raghu H, Sivakumar R, Naranatt PP, Chandran B. RhoA-GTPase facilitates entry of Kaposi's sarcoma-associated herpesvirus into adherent target cells in a Src-dependent manner.
J Virol 2006; 80: 11432-46. PMID: 17005646
- Veettil MV, Sadagopan S, Sharma-Walia N, Wang FZ, Raghu H, Varga L, Chandran B. Kaposi's sarcoma-associated herpesvirus forms a multimolecular complex of integrins (alphaVbeta5, alphaVbeta3, and alpha3beta1) and CD98-xCT during infection of human dermal microvascular endothelial cells, and CD98-xCT is essential for the postentry stage of infection. J Virol 2008; 82: 12126-44. PMID: 18829766
- Wang H, Tamba M, Kimata M, Sakamoto K, Bannai S, Sato H. Expression of the activity of cystine/glutamate exchange transporter, system x(c)(-), by xCT and rBAT.
Biochem Biophys Res Commun 2003; 305: 611-8. PMID: 12763038
- Zeng Y, Li Y, Chen RS, He X, Yang L, Li W. Overexpression of xCT induces up-regulation of 14-3-3beta in Kaposi's sarcoma. Biosci Rep 2010; 30: 277-83. PMID: 20100173
EDIT HISTORY:
Created by Wei Li, 08/18/2005
Updated by Wei Li, 04/06/2006
Updated by Wei Li, 12/28/2006
Updated by Wei Li, 08/20/2008
Updated by Wei Li, 03/12/2009
Updated by Wei Li, 05/29/2011
(1) Biological process: amino acid transport.
(2) Pheomelanin production.
(3) Oxidative stress.
(4) Belongs to the amino acid-polyamine-organocation (APC) superfamily. L-type amino acid transporter (LAT) (TC 2.A.3.8) family.
Location
Integral membrane protein. Transport system xc- was found in plasma membrane of cultured mammalian cells (Sato, et al (1999)).
Interaction
SLC7A11 (xCT), together with SLC3A2 (4F2hc, or CD98) linked by a disulfide bond at residue C158, encodes the heterodimeric amino acid transport system x(c)(-), which mediates cystine-glutamate exchange and thereby regulates intracellular glutathione levels. System x(c)- activity is important to maintain both intracellular glutathione levels and the redox balance between cystine and cysteine in the extracellular milieu. rBAT can replace 4F2hc in the expression of the activity of system x(c)(-) (Wang, et al).
In mouse NIH3T3 cells, the activity of system x(c)(-) and xCT mRNA is induced not only by deprivation of cystine but also by deprivation of other amino acids via a genomic cis-element termed amino acid response element (AARE) (Sato, et al (2004)). Overexpression of Nrf-2 in astrocytes specifically increases expression of the xCT cystine/glutamate antiporter and other thiol synthesis-related proteins, to promote cell survival (Qiang, et al). Cys(327) is a functionally important residue accessible to the aqueous extracellular environment and is structurally linked to the permeation pathway and/or the substrate binding site (Jimenez-Vidal, et al).
A CD44 variant (CD44v) interacts with xCT and controls the intracellular level of reduced glutathione (GSH). Ablation of CD44 induced loss of xCT from the cell surface and suppressed tumor growth in a transgenic mouse model of gastric cancer. It also induced activation of p38(MAPK), a downstream target of ROS, and expression of the gene for the cell cycle inhibitor p21(CIP1/WAF1)(Ishimoto, et al). xCT has been identified as a receptor mediating KSHV cell fusion (Kaleeba, et al).
miR-26b, which is down-regulated in human breast cancer specimens and cell lines, impairs viability and triggers apoptosis of human breast cancer MCF7 cells. SLC7A11 is identified as a direct target of miR-26b and its expression is remarkably increased in both breast cancer cell lines and clinical samples (Liu, et al).
Pathway
System x(c)(-) is a sodium-independent, high-affinity transporter of anionic amino acids with high specificity for anionic form of cystine and glutamate. Chintala, et al have reported that Slc7a11 regulates the pheomelanin production and cell proliferation. In melanosomes, dopaquinone reacts with cysteine to yield cysteinldopa, which is a precursor of pheomelanin. Cysteine is a component of the tripeptide glutathione. Glutathione metabolism pathway is described in KEGG Pathways.
MUTATION
Allele or SNP
No mutation has been reported in 32 human HPS patients who lack mutations in the seven known HPS genes (Chintala, et al).
SNPs deposited in dbSNP.
Distribution
(none)
Effect
(none)
PHENOTYPE
SLC7A11 is described in OMIM 607933.
Cystine uptake in human cancer cells is largely mediated by system Xc. xCT is relatively highly expressed in lung, colon, and central nervous system cancer cells (Huang, et al). Modulation of xCT activity is possiblely untilized in cancer therapy. Pharmacologic inhibition of system Xc remarkably inhibits the proliferation of several types of cancer cells, including lymphoma, glioma, prostate and breast cancer (Chung, et al; Doxsee, et al; Gout, et al; Narang, et al). This is thought be mediated by the depletion of GSH, which results in an increase of reactive oxygen species (ROS), and ultimately leads to caspase-dependent apoptosis (Doxsee, et al; Sakakura, et al). The apoptosis is likely mediated by c-Jun N-terminal kinase (JNK) activation. The JNK activation triggers both a caspase-dependent (caspases-9 and -3) and an ER stress-mediated (eIF2 and CHOP) pathway to induce apoptosis (Qiao, et al). In the absence of phospho-eIF2alpha, xCT expression is down-regulated that is mediated by nuclear factor ATF4, implicating the role eIF2alpha-ATF4-xCT module in neurodegeneration (Lewerenz, et al).
Disruption of xCT enhanced homotypic cell-cell adhesion and attenuated cell-extracellular matrix adhesion in an esophageal cancer cell line, KYSE150. SASP significantly inhibited both cell invasion of KYSE150 in vitro and its experimental metastasis in nude mice. Caveolin-1 was upregulated and beta-catenin was recruited to the plasma membrane when xCT was deficient, which were followed by the inhibition of beta-catenin transcriptional activity. Upregulation of caveolin-1 and inhibition of tumor cell invasion were mediated by reactive oxygen species-induced p38 MAPK activation (Chen, et al). Savaskan et al show that genetic or pharmacological inhibition of the glutamate transporter Xc system in gliomas leads to abrogated neurodegeneration, attenuated perifocal edema and prolonged survival. xCT deficiency causes unremitting inflammation because of the impaired survival of activated macrophages at the inflammatory site (Nabeyama, et al).
Kaposi's sarcoma-associated herpesvirus (KSHV, human herpesvirus 8) is the causative agent of Kaposi's sarcoma and other lymphoproliferative syndromes often associated with HIV/AIDS. xCT mediates the entry of KSHV which implicates its involvement in the infectious process of this pathogen (Kaleeba, et al; Veettil, et al (2006); Rappocciolo, et al). KSHV interacts with functionally related integrins (alphaVbeta3, alpha3beta1, and alphaVbeta5) and CD98/xCT molecules in a temporal fashion to form a multimolecular complex during the early stages of endothelial cell infection, probably mediating multiple roles in entry, signal transduction, and viral-gene expression (Veettil, et al (2008)). xCT was overexpressed in KS tissues and HHV-8-positive BCBL-1 cells. 14-3-3beta is a downstream effector of xCT in KS to mediate the cell proliferation (Zeng, et al). KSHV-encoded microRNAs (KSHV miRNAs) upregulate xCT expression by macrophages and endothelial cells, largely through miR-K12-11 suppression of BACH-1-a negative regulator of transcription recognizing antioxidant response elements within gene promoters (Qin, et al).
REFERENCE
- Chen RS, Song YM, Zhou ZY, Tong T, Li Y, Fu M, Guo XL, Dong LJ, He X, Qiao HX, Zhan QM, Li W. Disruption of xCT inhibits cancer cell metastasis via the caveolin-1/beta-catenin pathway. Oncogene 2009; 28: 599-609. PMID: 19015640
- Chintala S, Li W, Lamoreux ML, Ito S, Wakamatsu K, Sviderskaya EV, Bennett DC, Park YM, Gahl WA, Huizing M, Spritz RA, Ben S, Novak EK, Tan J, Swank RT.
Slc7a11 gene controls production of pheomelanin pigment and proliferation of cultured cells. Proc Natl Acad Sci U S A 2005; 102: 10964-9. PMID: 16037214
- Chung WJ, Lyons SA, Nelson GM, Hamza H, Gladson CL, Gillespie GY, Sontheimer H. Inhibition of cystine uptake disrupts the growth of primary brain tumors.
J Neurosci 2005; 25: 7101-10.
PMID: 16079392
- Doxsee DW, Gout PW, Kurita T, Lo M, Buckley AR, Wang Y, Xue H, Karp CM, Cutz JC, Cunha GR, Wang YZ. Sulfasalazine-induced cystine starvation: potential use for prostate cancer therapy. Prostate 2007; 67: 162-71.
PMID: 17075799
- Dun Y, Mysona B, Van Ells T, Amarnath L, Shamsul Ola M, Ganapathy V, Smith SB. Expression of the cystine-glutamate exchanger (x(c) (-)) in retinal ganglion cells and regulation by nitric oxide and oxidative stress. Cell Tissue Res 2006; 324: 189-202. PMID: 16444546
- Gasol E, Jimenez-Vidal M, Chillaron J, Zorzano A, Palacin M. Membrane topology of system xc- light subunit reveals a re-entrant loop with substrate-restricted accessibility.
J Biol Chem 2004; 279: 31228-36. PMID: 15151999
- Gout PW, Buckley AR, Simms CR, Bruchovsky N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the x(c)- cystine transporter: a new action for an old drug. Leukemia. 2001; 15: 1633-40.PMID: 11587223
- Huang Y, Dai Z, Barbacioru C, Sad¨¦e W. Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity and chemoresistance.Cancer Res 2005; 65: 7446-54.
PMID: 16103098
- Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, Masuko T, Shimizu T, Ishikawa T, Kai K, Takahashi E, Imamura Y, Baba Y, Ohmura M, Suematsu M, Baba H, Saya H. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 2011; 19: 387-400. PMID: 21397861
- Jimenez-Vidal M, Gasol E, Zorzano A, Nunes V, Palacin M, Chillaron J. Thiol modification of cysteine 327 in the eighth transmembrane domain of the light subunit xCT of the heteromeric cystine/glutamate antiporter suggests close proximity to the substrate binding site/permeation pathway.
J Biol Chem 2004; 279: 11214-21. PMID: 14722095
- Kaleeba JA, Berger EA. Kaposi's sarcoma-associated herpesvirus fusion-entry receptor: cystine transporter xCT. Science 2006; 311: 1921-4. PMID: 16574866
- Lewerenz J, Maher P. Basal levels of eIF2alpha phosphorylation determine cellular antioxidant status by regulating ATF4 and xCT expression. J Biol Chem 2009; 284: 1106-15. PMID: 19017641
- Liu XX, Li XJ, Zhang B, Liang YJ, Zhou CX, Cao DX, He M, Chen GQ, He JR, Zhao Q.
MicroRNA-26b is underexpressed in human breast cancer and induces cell apoptosis by targeting SLC7A11. FEBS Lett 2011; 585: 1363-7. PMID: 21510944
- Nabeyama A, Kurita A, Asano K, Miyake Y, Yasuda T, Miura I, Nishitai G, Arakawa S, Shimizu S, Wakana S, Yoshida H, Tanaka M. xCT deficiency accelerates chemically induced tumorigenesis. Proc Natl Acad Sci U S A 2010; 107: 6436-41.
PMID: 20308543
- Narang VS, Pauletti GM, Gout PW, Buckley DJ, Buckley AR. Suppression of cystine uptake by sulfasalazine inhibits proliferation of human mammary carcinoma cells.
Anticancer Res 2003; 23: 4571-9.
PMID: 14981898
- Rappocciolo G, Hensler HR, Jais M, Reinhart TA, Pegu A, Jenkins FJ, Rinaldo CR. Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC-SIGN. J Virol 2008; 82: 4793-806.
PMID: 18337571
- Qiang W, Cahill JM, Liu J, Kuang X, Liu N, Scofield VL, Voorhees JR, Reid AJ, Yan M, Lynn WS, Wong PK. Activation of transcription factor Nrf-2 and its downstream targets in response to moloney murine leukemia virus ts1-induced thiol depletion and oxidative stress in astrocytes. J Virol 2004; 78: 11926-38.
PMID: 15479833
- Qiao HX, Hao CJ, Li Y, He X, Chen RS, Cui J, Xu ZH, Li W. JNK activation mediates the apoptosis of xCT-deficient cells. Biochem Biophys Res Commun 2008; 370: 584-8. PMID: 18395005
- Qin Z, Freitas E, Sullivan R, Mohan S, Bacelieri R, Branch D, Romano M, Kearney P, Oates J, Plaisance K, Renne R, Kaleeba J, Parsons C. Upregulation of xCT by KSHV-encoded microRNAs facilitates KSHV dissemination and persistence in an environment of oxidative stress. PLoS Pathog 2010; 6: e1000742. PMID: 20126446
- Sakakura Y, Sato H, Shiiya A, Tamba M, Sagara J, Matsuda M, Okamura N, Makino N, Bannai S.Expression and function of cystine/glutamate transporter in neutrophils. J Leukoc Biol 2007; 81: 974-82. PMID: 17200146
- Sasaki H, Sato H, Kuriyama-Matsumura K, Sato K, Maebara K, Wang H, Tamba M, Itoh K, Yamamoto M, Bannai S. Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J Biol Chem 2002; 277: 44765-71. PMID: 12235164
- Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins.
J Biol Chem 1999; 274:11455-8. PMID: 10206947
- Sato H, Tamba M, Kuriyama-Matsumura K, Okuno S, Bannai S. Molecular cloning and expression of human xCT, the light chain of amino acid transport system xc-.
Antioxid Redox Signal. 2000; 2:665-71. PMID: 11213471
- Sato H, Tamba M, Okuno S, Sato K, Keino-Masu K, Masu M, Bannai S. Distribution of cystine/glutamate exchange transporter, system x(c)-, in the mouse brain.
J Neurosci 2002; 22: 8028-33.PMID: 12223556
- Sato H, Nomura S, Maebara K, Sato K, Tamba M, Bannai S. Transcriptional control of cystine/glutamate transporter gene by amino acid deprivation.
Biochem Biophys Res Commun 2004; 325: 109-16. PMID: 155522208
- Savaskan NE, Heckel A, Hahnen E, Engelhorn T, Doerfler A, Ganslandt O, Nimsky C, Buchfelder M, Ey¨ąpoglu IY. Small interfering RNA-mediated xCT silencing in gliomas inhibits neurodegeneration and alleviates brain edema. Nat Med 2008;14: 629-32.PMID: 18469825
- Veettil MV, Sharma-Walia N, Sadagopan S, Raghu H, Sivakumar R, Naranatt PP, Chandran B. RhoA-GTPase facilitates entry of Kaposi's sarcoma-associated herpesvirus into adherent target cells in a Src-dependent manner.
J Virol 2006; 80: 11432-46. PMID: 17005646
- Veettil MV, Sadagopan S, Sharma-Walia N, Wang FZ, Raghu H, Varga L, Chandran B. Kaposi's sarcoma-associated herpesvirus forms a multimolecular complex of integrins (alphaVbeta5, alphaVbeta3, and alpha3beta1) and CD98-xCT during infection of human dermal microvascular endothelial cells, and CD98-xCT is essential for the postentry stage of infection. J Virol 2008; 82: 12126-44. PMID: 18829766
- Wang H, Tamba M, Kimata M, Sakamoto K, Bannai S, Sato H. Expression of the activity of cystine/glutamate exchange transporter, system x(c)(-), by xCT and rBAT.
Biochem Biophys Res Commun 2003; 305: 611-8. PMID: 12763038
- Zeng Y, Li Y, Chen RS, He X, Yang L, Li W. Overexpression of xCT induces up-regulation of 14-3-3beta in Kaposi's sarcoma. Biosci Rep 2010; 30: 277-83. PMID: 20100173
EDIT HISTORY:
Created by Wei Li, 08/18/2005
Updated by Wei Li, 04/06/2006
Updated by Wei Li, 12/28/2006
Updated by Wei Li, 08/20/2008
Updated by Wei Li, 03/12/2009
Updated by Wei Li, 05/29/2011
Integral membrane protein. Transport system xc- was found in plasma membrane of cultured mammalian cells (Sato, et al (1999)).
Interaction
SLC7A11 (xCT), together with SLC3A2 (4F2hc, or CD98) linked by a disulfide bond at residue C158, encodes the heterodimeric amino acid transport system x(c)(-), which mediates cystine-glutamate exchange and thereby regulates intracellular glutathione levels. System x(c)- activity is important to maintain both intracellular glutathione levels and the redox balance between cystine and cysteine in the extracellular milieu. rBAT can replace 4F2hc in the expression of the activity of system x(c)(-) (Wang, et al).
In mouse NIH3T3 cells, the activity of system x(c)(-) and xCT mRNA is induced not only by deprivation of cystine but also by deprivation of other amino acids via a genomic cis-element termed amino acid response element (AARE) (Sato, et al (2004)). Overexpression of Nrf-2 in astrocytes specifically increases expression of the xCT cystine/glutamate antiporter and other thiol synthesis-related proteins, to promote cell survival (Qiang, et al). Cys(327) is a functionally important residue accessible to the aqueous extracellular environment and is structurally linked to the permeation pathway and/or the substrate binding site (Jimenez-Vidal, et al).
A CD44 variant (CD44v) interacts with xCT and controls the intracellular level of reduced glutathione (GSH). Ablation of CD44 induced loss of xCT from the cell surface and suppressed tumor growth in a transgenic mouse model of gastric cancer. It also induced activation of p38(MAPK), a downstream target of ROS, and expression of the gene for the cell cycle inhibitor p21(CIP1/WAF1)(Ishimoto, et al). xCT has been identified as a receptor mediating KSHV cell fusion (Kaleeba, et al).
miR-26b, which is down-regulated in human breast cancer specimens and cell lines, impairs viability and triggers apoptosis of human breast cancer MCF7 cells. SLC7A11 is identified as a direct target of miR-26b and its expression is remarkably increased in both breast cancer cell lines and clinical samples (Liu, et al).
Pathway
System x(c)(-) is a sodium-independent, high-affinity transporter of anionic amino acids with high specificity for anionic form of cystine and glutamate. Chintala, et al have reported that Slc7a11 regulates the pheomelanin production and cell proliferation. In melanosomes, dopaquinone reacts with cysteine to yield cysteinldopa, which is a precursor of pheomelanin. Cysteine is a component of the tripeptide glutathione. Glutathione metabolism pathway is described in KEGG Pathways.
MUTATION
Allele or SNP
No mutation has been reported in 32 human HPS patients who lack mutations in the seven known HPS genes (Chintala, et al).
SNPs deposited in dbSNP.
Distribution
(none)
Effect
(none)
PHENOTYPE
SLC7A11 is described in OMIM 607933.
Cystine uptake in human cancer cells is largely mediated by system Xc. xCT is relatively highly expressed in lung, colon, and central nervous system cancer cells (Huang, et al). Modulation of xCT activity is possiblely untilized in cancer therapy. Pharmacologic inhibition of system Xc remarkably inhibits the proliferation of several types of cancer cells, including lymphoma, glioma, prostate and breast cancer (Chung, et al; Doxsee, et al; Gout, et al; Narang, et al). This is thought be mediated by the depletion of GSH, which results in an increase of reactive oxygen species (ROS), and ultimately leads to caspase-dependent apoptosis (Doxsee, et al; Sakakura, et al). The apoptosis is likely mediated by c-Jun N-terminal kinase (JNK) activation. The JNK activation triggers both a caspase-dependent (caspases-9 and -3) and an ER stress-mediated (eIF2 and CHOP) pathway to induce apoptosis (Qiao, et al). In the absence of phospho-eIF2alpha, xCT expression is down-regulated that is mediated by nuclear factor ATF4, implicating the role eIF2alpha-ATF4-xCT module in neurodegeneration (Lewerenz, et al).
Disruption of xCT enhanced homotypic cell-cell adhesion and attenuated cell-extracellular matrix adhesion in an esophageal cancer cell line, KYSE150. SASP significantly inhibited both cell invasion of KYSE150 in vitro and its experimental metastasis in nude mice. Caveolin-1 was upregulated and beta-catenin was recruited to the plasma membrane when xCT was deficient, which were followed by the inhibition of beta-catenin transcriptional activity. Upregulation of caveolin-1 and inhibition of tumor cell invasion were mediated by reactive oxygen species-induced p38 MAPK activation (Chen, et al). Savaskan et al show that genetic or pharmacological inhibition of the glutamate transporter Xc system in gliomas leads to abrogated neurodegeneration, attenuated perifocal edema and prolonged survival. xCT deficiency causes unremitting inflammation because of the impaired survival of activated macrophages at the inflammatory site (Nabeyama, et al).
Kaposi's sarcoma-associated herpesvirus (KSHV, human herpesvirus 8) is the causative agent of Kaposi's sarcoma and other lymphoproliferative syndromes often associated with HIV/AIDS. xCT mediates the entry of KSHV which implicates its involvement in the infectious process of this pathogen (Kaleeba, et al; Veettil, et al (2006); Rappocciolo, et al). KSHV interacts with functionally related integrins (alphaVbeta3, alpha3beta1, and alphaVbeta5) and CD98/xCT molecules in a temporal fashion to form a multimolecular complex during the early stages of endothelial cell infection, probably mediating multiple roles in entry, signal transduction, and viral-gene expression (Veettil, et al (2008)). xCT was overexpressed in KS tissues and HHV-8-positive BCBL-1 cells. 14-3-3beta is a downstream effector of xCT in KS to mediate the cell proliferation (Zeng, et al). KSHV-encoded microRNAs (KSHV miRNAs) upregulate xCT expression by macrophages and endothelial cells, largely through miR-K12-11 suppression of BACH-1-a negative regulator of transcription recognizing antioxidant response elements within gene promoters (Qin, et al).
REFERENCE
- Chen RS, Song YM, Zhou ZY, Tong T, Li Y, Fu M, Guo XL, Dong LJ, He X, Qiao HX, Zhan QM, Li W. Disruption of xCT inhibits cancer cell metastasis via the caveolin-1/beta-catenin pathway. Oncogene 2009; 28: 599-609. PMID: 19015640
- Chintala S, Li W, Lamoreux ML, Ito S, Wakamatsu K, Sviderskaya EV, Bennett DC, Park YM, Gahl WA, Huizing M, Spritz RA, Ben S, Novak EK, Tan J, Swank RT.
Slc7a11 gene controls production of pheomelanin pigment and proliferation of cultured cells. Proc Natl Acad Sci U S A 2005; 102: 10964-9. PMID: 16037214
- Chung WJ, Lyons SA, Nelson GM, Hamza H, Gladson CL, Gillespie GY, Sontheimer H. Inhibition of cystine uptake disrupts the growth of primary brain tumors.
J Neurosci 2005; 25: 7101-10.
PMID: 16079392
- Doxsee DW, Gout PW, Kurita T, Lo M, Buckley AR, Wang Y, Xue H, Karp CM, Cutz JC, Cunha GR, Wang YZ. Sulfasalazine-induced cystine starvation: potential use for prostate cancer therapy. Prostate 2007; 67: 162-71.
PMID: 17075799
- Dun Y, Mysona B, Van Ells T, Amarnath L, Shamsul Ola M, Ganapathy V, Smith SB. Expression of the cystine-glutamate exchanger (x(c) (-)) in retinal ganglion cells and regulation by nitric oxide and oxidative stress. Cell Tissue Res 2006; 324: 189-202. PMID: 16444546
- Gasol E, Jimenez-Vidal M, Chillaron J, Zorzano A, Palacin M. Membrane topology of system xc- light subunit reveals a re-entrant loop with substrate-restricted accessibility.
J Biol Chem 2004; 279: 31228-36. PMID: 15151999
- Gout PW, Buckley AR, Simms CR, Bruchovsky N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the x(c)- cystine transporter: a new action for an old drug. Leukemia. 2001; 15: 1633-40.PMID: 11587223
- Huang Y, Dai Z, Barbacioru C, Sad¨¦e W. Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity and chemoresistance.Cancer Res 2005; 65: 7446-54.
PMID: 16103098
- Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, Masuko T, Shimizu T, Ishikawa T, Kai K, Takahashi E, Imamura Y, Baba Y, Ohmura M, Suematsu M, Baba H, Saya H. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 2011; 19: 387-400. PMID: 21397861
- Jimenez-Vidal M, Gasol E, Zorzano A, Nunes V, Palacin M, Chillaron J. Thiol modification of cysteine 327 in the eighth transmembrane domain of the light subunit xCT of the heteromeric cystine/glutamate antiporter suggests close proximity to the substrate binding site/permeation pathway.
J Biol Chem 2004; 279: 11214-21. PMID: 14722095
- Kaleeba JA, Berger EA. Kaposi's sarcoma-associated herpesvirus fusion-entry receptor: cystine transporter xCT. Science 2006; 311: 1921-4. PMID: 16574866
- Lewerenz J, Maher P. Basal levels of eIF2alpha phosphorylation determine cellular antioxidant status by regulating ATF4 and xCT expression. J Biol Chem 2009; 284: 1106-15. PMID: 19017641
- Liu XX, Li XJ, Zhang B, Liang YJ, Zhou CX, Cao DX, He M, Chen GQ, He JR, Zhao Q.
MicroRNA-26b is underexpressed in human breast cancer and induces cell apoptosis by targeting SLC7A11. FEBS Lett 2011; 585: 1363-7. PMID: 21510944
- Nabeyama A, Kurita A, Asano K, Miyake Y, Yasuda T, Miura I, Nishitai G, Arakawa S, Shimizu S, Wakana S, Yoshida H, Tanaka M. xCT deficiency accelerates chemically induced tumorigenesis. Proc Natl Acad Sci U S A 2010; 107: 6436-41.
PMID: 20308543
- Narang VS, Pauletti GM, Gout PW, Buckley DJ, Buckley AR. Suppression of cystine uptake by sulfasalazine inhibits proliferation of human mammary carcinoma cells.
Anticancer Res 2003; 23: 4571-9.
PMID: 14981898
- Rappocciolo G, Hensler HR, Jais M, Reinhart TA, Pegu A, Jenkins FJ, Rinaldo CR. Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC-SIGN. J Virol 2008; 82: 4793-806.
PMID: 18337571
- Qiang W, Cahill JM, Liu J, Kuang X, Liu N, Scofield VL, Voorhees JR, Reid AJ, Yan M, Lynn WS, Wong PK. Activation of transcription factor Nrf-2 and its downstream targets in response to moloney murine leukemia virus ts1-induced thiol depletion and oxidative stress in astrocytes. J Virol 2004; 78: 11926-38.
PMID: 15479833
- Qiao HX, Hao CJ, Li Y, He X, Chen RS, Cui J, Xu ZH, Li W. JNK activation mediates the apoptosis of xCT-deficient cells. Biochem Biophys Res Commun 2008; 370: 584-8. PMID: 18395005
- Qin Z, Freitas E, Sullivan R, Mohan S, Bacelieri R, Branch D, Romano M, Kearney P, Oates J, Plaisance K, Renne R, Kaleeba J, Parsons C. Upregulation of xCT by KSHV-encoded microRNAs facilitates KSHV dissemination and persistence in an environment of oxidative stress. PLoS Pathog 2010; 6: e1000742. PMID: 20126446
- Sakakura Y, Sato H, Shiiya A, Tamba M, Sagara J, Matsuda M, Okamura N, Makino N, Bannai S.Expression and function of cystine/glutamate transporter in neutrophils. J Leukoc Biol 2007; 81: 974-82. PMID: 17200146
- Sasaki H, Sato H, Kuriyama-Matsumura K, Sato K, Maebara K, Wang H, Tamba M, Itoh K, Yamamoto M, Bannai S. Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J Biol Chem 2002; 277: 44765-71. PMID: 12235164
- Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins.
J Biol Chem 1999; 274:11455-8. PMID: 10206947
- Sato H, Tamba M, Kuriyama-Matsumura K, Okuno S, Bannai S. Molecular cloning and expression of human xCT, the light chain of amino acid transport system xc-.
Antioxid Redox Signal. 2000; 2:665-71. PMID: 11213471
- Sato H, Tamba M, Okuno S, Sato K, Keino-Masu K, Masu M, Bannai S. Distribution of cystine/glutamate exchange transporter, system x(c)-, in the mouse brain.
J Neurosci 2002; 22: 8028-33.PMID: 12223556
- Sato H, Nomura S, Maebara K, Sato K, Tamba M, Bannai S. Transcriptional control of cystine/glutamate transporter gene by amino acid deprivation.
Biochem Biophys Res Commun 2004; 325: 109-16. PMID: 155522208
- Savaskan NE, Heckel A, Hahnen E, Engelhorn T, Doerfler A, Ganslandt O, Nimsky C, Buchfelder M, Ey¨ąpoglu IY. Small interfering RNA-mediated xCT silencing in gliomas inhibits neurodegeneration and alleviates brain edema. Nat Med 2008;14: 629-32.PMID: 18469825
- Veettil MV, Sharma-Walia N, Sadagopan S, Raghu H, Sivakumar R, Naranatt PP, Chandran B. RhoA-GTPase facilitates entry of Kaposi's sarcoma-associated herpesvirus into adherent target cells in a Src-dependent manner.
J Virol 2006; 80: 11432-46. PMID: 17005646
- Veettil MV, Sadagopan S, Sharma-Walia N, Wang FZ, Raghu H, Varga L, Chandran B. Kaposi's sarcoma-associated herpesvirus forms a multimolecular complex of integrins (alphaVbeta5, alphaVbeta3, and alpha3beta1) and CD98-xCT during infection of human dermal microvascular endothelial cells, and CD98-xCT is essential for the postentry stage of infection. J Virol 2008; 82: 12126-44. PMID: 18829766
- Wang H, Tamba M, Kimata M, Sakamoto K, Bannai S, Sato H. Expression of the activity of cystine/glutamate exchange transporter, system x(c)(-), by xCT and rBAT.
Biochem Biophys Res Commun 2003; 305: 611-8. PMID: 12763038
- Zeng Y, Li Y, Chen RS, He X, Yang L, Li W. Overexpression of xCT induces up-regulation of 14-3-3beta in Kaposi's sarcoma. Biosci Rep 2010; 30: 277-83. PMID: 20100173
EDIT HISTORY:
Created by Wei Li, 08/18/2005
Updated by Wei Li, 04/06/2006
Updated by Wei Li, 12/28/2006
Updated by Wei Li, 08/20/2008
Updated by Wei Li, 03/12/2009
Updated by Wei Li, 05/29/2011
SLC7A11 (xCT), together with SLC3A2 (4F2hc, or CD98) linked by a disulfide bond at residue C158, encodes the heterodimeric amino acid transport system x(c)(-), which mediates cystine-glutamate exchange and thereby regulates intracellular glutathione levels. System x(c)- activity is important to maintain both intracellular glutathione levels and the redox balance between cystine and cysteine in the extracellular milieu. rBAT can replace 4F2hc in the expression of the activity of system x(c)(-) (Wang, et al).
In mouse NIH3T3 cells, the activity of system x(c)(-) and xCT mRNA is induced not only by deprivation of cystine but also by deprivation of other amino acids via a genomic cis-element termed amino acid response element (AARE) (Sato, et al (2004)). Overexpression of Nrf-2 in astrocytes specifically increases expression of the xCT cystine/glutamate antiporter and other thiol synthesis-related proteins, to promote cell survival (Qiang, et al). Cys(327) is a functionally important residue accessible to the aqueous extracellular environment and is structurally linked to the permeation pathway and/or the substrate binding site (Jimenez-Vidal, et al).
A CD44 variant (CD44v) interacts with xCT and controls the intracellular level of reduced glutathione (GSH). Ablation of CD44 induced loss of xCT from the cell surface and suppressed tumor growth in a transgenic mouse model of gastric cancer. It also induced activation of p38(MAPK), a downstream target of ROS, and expression of the gene for the cell cycle inhibitor p21(CIP1/WAF1)(Ishimoto, et al). xCT has been identified as a receptor mediating KSHV cell fusion (Kaleeba, et al).
miR-26b, which is down-regulated in human breast cancer specimens and cell lines, impairs viability and triggers apoptosis of human breast cancer MCF7 cells. SLC7A11 is identified as a direct target of miR-26b and its expression is remarkably increased in both breast cancer cell lines and clinical samples (Liu, et al).
Pathway
System x(c)(-) is a sodium-independent, high-affinity transporter of anionic amino acids with high specificity for anionic form of cystine and glutamate. Chintala, et al have reported that Slc7a11 regulates the pheomelanin production and cell proliferation. In melanosomes, dopaquinone reacts with cysteine to yield cysteinldopa, which is a precursor of pheomelanin. Cysteine is a component of the tripeptide glutathione. Glutathione metabolism pathway is described in KEGG Pathways.
MUTATION
Allele or SNP
No mutation has been reported in 32 human HPS patients who lack mutations in the seven known HPS genes (Chintala, et al).
SNPs deposited in dbSNP.
Distribution
(none)
Effect
(none)
PHENOTYPE
SLC7A11 is described in OMIM 607933.
Cystine uptake in human cancer cells is largely mediated by system Xc. xCT is relatively highly expressed in lung, colon, and central nervous system cancer cells (Huang, et al). Modulation of xCT activity is possiblely untilized in cancer therapy. Pharmacologic inhibition of system Xc remarkably inhibits the proliferation of several types of cancer cells, including lymphoma, glioma, prostate and breast cancer (Chung, et al; Doxsee, et al; Gout, et al; Narang, et al). This is thought be mediated by the depletion of GSH, which results in an increase of reactive oxygen species (ROS), and ultimately leads to caspase-dependent apoptosis (Doxsee, et al; Sakakura, et al). The apoptosis is likely mediated by c-Jun N-terminal kinase (JNK) activation. The JNK activation triggers both a caspase-dependent (caspases-9 and -3) and an ER stress-mediated (eIF2 and CHOP) pathway to induce apoptosis (Qiao, et al). In the absence of phospho-eIF2alpha, xCT expression is down-regulated that is mediated by nuclear factor ATF4, implicating the role eIF2alpha-ATF4-xCT module in neurodegeneration (Lewerenz, et al).
Disruption of xCT enhanced homotypic cell-cell adhesion and attenuated cell-extracellular matrix adhesion in an esophageal cancer cell line, KYSE150. SASP significantly inhibited both cell invasion of KYSE150 in vitro and its experimental metastasis in nude mice. Caveolin-1 was upregulated and beta-catenin was recruited to the plasma membrane when xCT was deficient, which were followed by the inhibition of beta-catenin transcriptional activity. Upregulation of caveolin-1 and inhibition of tumor cell invasion were mediated by reactive oxygen species-induced p38 MAPK activation (Chen, et al). Savaskan et al show that genetic or pharmacological inhibition of the glutamate transporter Xc system in gliomas leads to abrogated neurodegeneration, attenuated perifocal edema and prolonged survival. xCT deficiency causes unremitting inflammation because of the impaired survival of activated macrophages at the inflammatory site (Nabeyama, et al).
Kaposi's sarcoma-associated herpesvirus (KSHV, human herpesvirus 8) is the causative agent of Kaposi's sarcoma and other lymphoproliferative syndromes often associated with HIV/AIDS. xCT mediates the entry of KSHV which implicates its involvement in the infectious process of this pathogen (Kaleeba, et al; Veettil, et al (2006); Rappocciolo, et al). KSHV interacts with functionally related integrins (alphaVbeta3, alpha3beta1, and alphaVbeta5) and CD98/xCT molecules in a temporal fashion to form a multimolecular complex during the early stages of endothelial cell infection, probably mediating multiple roles in entry, signal transduction, and viral-gene expression (Veettil, et al (2008)). xCT was overexpressed in KS tissues and HHV-8-positive BCBL-1 cells. 14-3-3beta is a downstream effector of xCT in KS to mediate the cell proliferation (Zeng, et al). KSHV-encoded microRNAs (KSHV miRNAs) upregulate xCT expression by macrophages and endothelial cells, largely through miR-K12-11 suppression of BACH-1-a negative regulator of transcription recognizing antioxidant response elements within gene promoters (Qin, et al).
REFERENCE
- Chen RS, Song YM, Zhou ZY, Tong T, Li Y, Fu M, Guo XL, Dong LJ, He X, Qiao HX, Zhan QM, Li W. Disruption of xCT inhibits cancer cell metastasis via the caveolin-1/beta-catenin pathway. Oncogene 2009; 28: 599-609. PMID: 19015640
- Chintala S, Li W, Lamoreux ML, Ito S, Wakamatsu K, Sviderskaya EV, Bennett DC, Park YM, Gahl WA, Huizing M, Spritz RA, Ben S, Novak EK, Tan J, Swank RT.
Slc7a11 gene controls production of pheomelanin pigment and proliferation of cultured cells. Proc Natl Acad Sci U S A 2005; 102: 10964-9. PMID: 16037214
- Chung WJ, Lyons SA, Nelson GM, Hamza H, Gladson CL, Gillespie GY, Sontheimer H. Inhibition of cystine uptake disrupts the growth of primary brain tumors.
J Neurosci 2005; 25: 7101-10.
PMID: 16079392
- Doxsee DW, Gout PW, Kurita T, Lo M, Buckley AR, Wang Y, Xue H, Karp CM, Cutz JC, Cunha GR, Wang YZ. Sulfasalazine-induced cystine starvation: potential use for prostate cancer therapy. Prostate 2007; 67: 162-71.
PMID: 17075799
- Dun Y, Mysona B, Van Ells T, Amarnath L, Shamsul Ola M, Ganapathy V, Smith SB. Expression of the cystine-glutamate exchanger (x(c) (-)) in retinal ganglion cells and regulation by nitric oxide and oxidative stress. Cell Tissue Res 2006; 324: 189-202. PMID: 16444546
- Gasol E, Jimenez-Vidal M, Chillaron J, Zorzano A, Palacin M. Membrane topology of system xc- light subunit reveals a re-entrant loop with substrate-restricted accessibility.
J Biol Chem 2004; 279: 31228-36. PMID: 15151999
- Gout PW, Buckley AR, Simms CR, Bruchovsky N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the x(c)- cystine transporter: a new action for an old drug. Leukemia. 2001; 15: 1633-40.PMID: 11587223
- Huang Y, Dai Z, Barbacioru C, Sad¨¦e W. Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity and chemoresistance.Cancer Res 2005; 65: 7446-54.
PMID: 16103098
- Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, Masuko T, Shimizu T, Ishikawa T, Kai K, Takahashi E, Imamura Y, Baba Y, Ohmura M, Suematsu M, Baba H, Saya H. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 2011; 19: 387-400. PMID: 21397861
- Jimenez-Vidal M, Gasol E, Zorzano A, Nunes V, Palacin M, Chillaron J. Thiol modification of cysteine 327 in the eighth transmembrane domain of the light subunit xCT of the heteromeric cystine/glutamate antiporter suggests close proximity to the substrate binding site/permeation pathway.
J Biol Chem 2004; 279: 11214-21. PMID: 14722095
- Kaleeba JA, Berger EA. Kaposi's sarcoma-associated herpesvirus fusion-entry receptor: cystine transporter xCT. Science 2006; 311: 1921-4. PMID: 16574866
- Lewerenz J, Maher P. Basal levels of eIF2alpha phosphorylation determine cellular antioxidant status by regulating ATF4 and xCT expression. J Biol Chem 2009; 284: 1106-15. PMID: 19017641
- Liu XX, Li XJ, Zhang B, Liang YJ, Zhou CX, Cao DX, He M, Chen GQ, He JR, Zhao Q.
MicroRNA-26b is underexpressed in human breast cancer and induces cell apoptosis by targeting SLC7A11. FEBS Lett 2011; 585: 1363-7. PMID: 21510944
- Nabeyama A, Kurita A, Asano K, Miyake Y, Yasuda T, Miura I, Nishitai G, Arakawa S, Shimizu S, Wakana S, Yoshida H, Tanaka M. xCT deficiency accelerates chemically induced tumorigenesis. Proc Natl Acad Sci U S A 2010; 107: 6436-41.
PMID: 20308543
- Narang VS, Pauletti GM, Gout PW, Buckley DJ, Buckley AR. Suppression of cystine uptake by sulfasalazine inhibits proliferation of human mammary carcinoma cells.
Anticancer Res 2003; 23: 4571-9.
PMID: 14981898
- Rappocciolo G, Hensler HR, Jais M, Reinhart TA, Pegu A, Jenkins FJ, Rinaldo CR. Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC-SIGN. J Virol 2008; 82: 4793-806.
PMID: 18337571
- Qiang W, Cahill JM, Liu J, Kuang X, Liu N, Scofield VL, Voorhees JR, Reid AJ, Yan M, Lynn WS, Wong PK. Activation of transcription factor Nrf-2 and its downstream targets in response to moloney murine leukemia virus ts1-induced thiol depletion and oxidative stress in astrocytes. J Virol 2004; 78: 11926-38.
PMID: 15479833
- Qiao HX, Hao CJ, Li Y, He X, Chen RS, Cui J, Xu ZH, Li W. JNK activation mediates the apoptosis of xCT-deficient cells. Biochem Biophys Res Commun 2008; 370: 584-8. PMID: 18395005
- Qin Z, Freitas E, Sullivan R, Mohan S, Bacelieri R, Branch D, Romano M, Kearney P, Oates J, Plaisance K, Renne R, Kaleeba J, Parsons C. Upregulation of xCT by KSHV-encoded microRNAs facilitates KSHV dissemination and persistence in an environment of oxidative stress. PLoS Pathog 2010; 6: e1000742. PMID: 20126446
- Sakakura Y, Sato H, Shiiya A, Tamba M, Sagara J, Matsuda M, Okamura N, Makino N, Bannai S.Expression and function of cystine/glutamate transporter in neutrophils. J Leukoc Biol 2007; 81: 974-82. PMID: 17200146
- Sasaki H, Sato H, Kuriyama-Matsumura K, Sato K, Maebara K, Wang H, Tamba M, Itoh K, Yamamoto M, Bannai S. Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J Biol Chem 2002; 277: 44765-71. PMID: 12235164
- Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins.
J Biol Chem 1999; 274:11455-8. PMID: 10206947
- Sato H, Tamba M, Kuriyama-Matsumura K, Okuno S, Bannai S. Molecular cloning and expression of human xCT, the light chain of amino acid transport system xc-.
Antioxid Redox Signal. 2000; 2:665-71. PMID: 11213471
- Sato H, Tamba M, Okuno S, Sato K, Keino-Masu K, Masu M, Bannai S. Distribution of cystine/glutamate exchange transporter, system x(c)-, in the mouse brain.
J Neurosci 2002; 22: 8028-33.PMID: 12223556
- Sato H, Nomura S, Maebara K, Sato K, Tamba M, Bannai S. Transcriptional control of cystine/glutamate transporter gene by amino acid deprivation.
Biochem Biophys Res Commun 2004; 325: 109-16. PMID: 155522208
- Savaskan NE, Heckel A, Hahnen E, Engelhorn T, Doerfler A, Ganslandt O, Nimsky C, Buchfelder M, Ey¨ąpoglu IY. Small interfering RNA-mediated xCT silencing in gliomas inhibits neurodegeneration and alleviates brain edema. Nat Med 2008;14: 629-32.PMID: 18469825
- Veettil MV, Sharma-Walia N, Sadagopan S, Raghu H, Sivakumar R, Naranatt PP, Chandran B. RhoA-GTPase facilitates entry of Kaposi's sarcoma-associated herpesvirus into adherent target cells in a Src-dependent manner.
J Virol 2006; 80: 11432-46. PMID: 17005646
- Veettil MV, Sadagopan S, Sharma-Walia N, Wang FZ, Raghu H, Varga L, Chandran B. Kaposi's sarcoma-associated herpesvirus forms a multimolecular complex of integrins (alphaVbeta5, alphaVbeta3, and alpha3beta1) and CD98-xCT during infection of human dermal microvascular endothelial cells, and CD98-xCT is essential for the postentry stage of infection. J Virol 2008; 82: 12126-44. PMID: 18829766
- Wang H, Tamba M, Kimata M, Sakamoto K, Bannai S, Sato H. Expression of the activity of cystine/glutamate exchange transporter, system x(c)(-), by xCT and rBAT.
Biochem Biophys Res Commun 2003; 305: 611-8. PMID: 12763038
- Zeng Y, Li Y, Chen RS, He X, Yang L, Li W. Overexpression of xCT induces up-regulation of 14-3-3beta in Kaposi's sarcoma. Biosci Rep 2010; 30: 277-83. PMID: 20100173
EDIT HISTORY:
Created by Wei Li, 08/18/2005
Updated by Wei Li, 04/06/2006
Updated by Wei Li, 12/28/2006
Updated by Wei Li, 08/20/2008
Updated by Wei Li, 03/12/2009
Updated by Wei Li, 05/29/2011
System x(c)(-) is a sodium-independent, high-affinity transporter of anionic amino acids with high specificity for anionic form of cystine and glutamate. Chintala, et al have reported that Slc7a11 regulates the pheomelanin production and cell proliferation. In melanosomes, dopaquinone reacts with cysteine to yield cysteinldopa, which is a precursor of pheomelanin. Cysteine is a component of the tripeptide glutathione. Glutathione metabolism pathway is described in KEGG Pathways.
MUTATION
Allele or SNP
No mutation has been reported in 32 human HPS patients who lack mutations in the seven known HPS genes (Chintala, et al).
SNPs deposited in dbSNP.
Distribution
(none)
Effect
(none)
PHENOTYPE
SLC7A11 is described in OMIM 607933.
Cystine uptake in human cancer cells is largely mediated by system Xc. xCT is relatively highly expressed in lung, colon, and central nervous system cancer cells (Huang, et al). Modulation of xCT activity is possiblely untilized in cancer therapy. Pharmacologic inhibition of system Xc remarkably inhibits the proliferation of several types of cancer cells, including lymphoma, glioma, prostate and breast cancer (Chung, et al; Doxsee, et al; Gout, et al; Narang, et al). This is thought be mediated by the depletion of GSH, which results in an increase of reactive oxygen species (ROS), and ultimately leads to caspase-dependent apoptosis (Doxsee, et al; Sakakura, et al). The apoptosis is likely mediated by c-Jun N-terminal kinase (JNK) activation. The JNK activation triggers both a caspase-dependent (caspases-9 and -3) and an ER stress-mediated (eIF2 and CHOP) pathway to induce apoptosis (Qiao, et al). In the absence of phospho-eIF2alpha, xCT expression is down-regulated that is mediated by nuclear factor ATF4, implicating the role eIF2alpha-ATF4-xCT module in neurodegeneration (Lewerenz, et al).
Disruption of xCT enhanced homotypic cell-cell adhesion and attenuated cell-extracellular matrix adhesion in an esophageal cancer cell line, KYSE150. SASP significantly inhibited both cell invasion of KYSE150 in vitro and its experimental metastasis in nude mice. Caveolin-1 was upregulated and beta-catenin was recruited to the plasma membrane when xCT was deficient, which were followed by the inhibition of beta-catenin transcriptional activity. Upregulation of caveolin-1 and inhibition of tumor cell invasion were mediated by reactive oxygen species-induced p38 MAPK activation (Chen, et al). Savaskan et al show that genetic or pharmacological inhibition of the glutamate transporter Xc system in gliomas leads to abrogated neurodegeneration, attenuated perifocal edema and prolonged survival. xCT deficiency causes unremitting inflammation because of the impaired survival of activated macrophages at the inflammatory site (Nabeyama, et al).
Kaposi's sarcoma-associated herpesvirus (KSHV, human herpesvirus 8) is the causative agent of Kaposi's sarcoma and other lymphoproliferative syndromes often associated with HIV/AIDS. xCT mediates the entry of KSHV which implicates its involvement in the infectious process of this pathogen (Kaleeba, et al; Veettil, et al (2006); Rappocciolo, et al). KSHV interacts with functionally related integrins (alphaVbeta3, alpha3beta1, and alphaVbeta5) and CD98/xCT molecules in a temporal fashion to form a multimolecular complex during the early stages of endothelial cell infection, probably mediating multiple roles in entry, signal transduction, and viral-gene expression (Veettil, et al (2008)). xCT was overexpressed in KS tissues and HHV-8-positive BCBL-1 cells. 14-3-3beta is a downstream effector of xCT in KS to mediate the cell proliferation (Zeng, et al). KSHV-encoded microRNAs (KSHV miRNAs) upregulate xCT expression by macrophages and endothelial cells, largely through miR-K12-11 suppression of BACH-1-a negative regulator of transcription recognizing antioxidant response elements within gene promoters (Qin, et al).
REFERENCE
- Chen RS, Song YM, Zhou ZY, Tong T, Li Y, Fu M, Guo XL, Dong LJ, He X, Qiao HX, Zhan QM, Li W. Disruption of xCT inhibits cancer cell metastasis via the caveolin-1/beta-catenin pathway. Oncogene 2009; 28: 599-609. PMID: 19015640
- Chintala S, Li W, Lamoreux ML, Ito S, Wakamatsu K, Sviderskaya EV, Bennett DC, Park YM, Gahl WA, Huizing M, Spritz RA, Ben S, Novak EK, Tan J, Swank RT.
Slc7a11 gene controls production of pheomelanin pigment and proliferation of cultured cells. Proc Natl Acad Sci U S A 2005; 102: 10964-9. PMID: 16037214
- Chung WJ, Lyons SA, Nelson GM, Hamza H, Gladson CL, Gillespie GY, Sontheimer H. Inhibition of cystine uptake disrupts the growth of primary brain tumors.
J Neurosci 2005; 25: 7101-10.
PMID: 16079392
- Doxsee DW, Gout PW, Kurita T, Lo M, Buckley AR, Wang Y, Xue H, Karp CM, Cutz JC, Cunha GR, Wang YZ. Sulfasalazine-induced cystine starvation: potential use for prostate cancer therapy. Prostate 2007; 67: 162-71.
PMID: 17075799
- Dun Y, Mysona B, Van Ells T, Amarnath L, Shamsul Ola M, Ganapathy V, Smith SB. Expression of the cystine-glutamate exchanger (x(c) (-)) in retinal ganglion cells and regulation by nitric oxide and oxidative stress. Cell Tissue Res 2006; 324: 189-202. PMID: 16444546
- Gasol E, Jimenez-Vidal M, Chillaron J, Zorzano A, Palacin M. Membrane topology of system xc- light subunit reveals a re-entrant loop with substrate-restricted accessibility.
J Biol Chem 2004; 279: 31228-36. PMID: 15151999
- Gout PW, Buckley AR, Simms CR, Bruchovsky N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the x(c)- cystine transporter: a new action for an old drug. Leukemia. 2001; 15: 1633-40.PMID: 11587223
- Huang Y, Dai Z, Barbacioru C, Sad¨¦e W. Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity and chemoresistance.Cancer Res 2005; 65: 7446-54.
PMID: 16103098
- Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, Masuko T, Shimizu T, Ishikawa T, Kai K, Takahashi E, Imamura Y, Baba Y, Ohmura M, Suematsu M, Baba H, Saya H. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 2011; 19: 387-400. PMID: 21397861
- Jimenez-Vidal M, Gasol E, Zorzano A, Nunes V, Palacin M, Chillaron J. Thiol modification of cysteine 327 in the eighth transmembrane domain of the light subunit xCT of the heteromeric cystine/glutamate antiporter suggests close proximity to the substrate binding site/permeation pathway.
J Biol Chem 2004; 279: 11214-21. PMID: 14722095
- Kaleeba JA, Berger EA. Kaposi's sarcoma-associated herpesvirus fusion-entry receptor: cystine transporter xCT. Science 2006; 311: 1921-4. PMID: 16574866
- Lewerenz J, Maher P. Basal levels of eIF2alpha phosphorylation determine cellular antioxidant status by regulating ATF4 and xCT expression. J Biol Chem 2009; 284: 1106-15. PMID: 19017641
- Liu XX, Li XJ, Zhang B, Liang YJ, Zhou CX, Cao DX, He M, Chen GQ, He JR, Zhao Q.
MicroRNA-26b is underexpressed in human breast cancer and induces cell apoptosis by targeting SLC7A11. FEBS Lett 2011; 585: 1363-7. PMID: 21510944
- Nabeyama A, Kurita A, Asano K, Miyake Y, Yasuda T, Miura I, Nishitai G, Arakawa S, Shimizu S, Wakana S, Yoshida H, Tanaka M. xCT deficiency accelerates chemically induced tumorigenesis. Proc Natl Acad Sci U S A 2010; 107: 6436-41.
PMID: 20308543
- Narang VS, Pauletti GM, Gout PW, Buckley DJ, Buckley AR. Suppression of cystine uptake by sulfasalazine inhibits proliferation of human mammary carcinoma cells.
Anticancer Res 2003; 23: 4571-9.
PMID: 14981898
- Rappocciolo G, Hensler HR, Jais M, Reinhart TA, Pegu A, Jenkins FJ, Rinaldo CR. Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC-SIGN. J Virol 2008; 82: 4793-806.
PMID: 18337571
- Qiang W, Cahill JM, Liu J, Kuang X, Liu N, Scofield VL, Voorhees JR, Reid AJ, Yan M, Lynn WS, Wong PK. Activation of transcription factor Nrf-2 and its downstream targets in response to moloney murine leukemia virus ts1-induced thiol depletion and oxidative stress in astrocytes. J Virol 2004; 78: 11926-38.
PMID: 15479833
- Qiao HX, Hao CJ, Li Y, He X, Chen RS, Cui J, Xu ZH, Li W. JNK activation mediates the apoptosis of xCT-deficient cells. Biochem Biophys Res Commun 2008; 370: 584-8. PMID: 18395005
- Qin Z, Freitas E, Sullivan R, Mohan S, Bacelieri R, Branch D, Romano M, Kearney P, Oates J, Plaisance K, Renne R, Kaleeba J, Parsons C. Upregulation of xCT by KSHV-encoded microRNAs facilitates KSHV dissemination and persistence in an environment of oxidative stress. PLoS Pathog 2010; 6: e1000742. PMID: 20126446
- Sakakura Y, Sato H, Shiiya A, Tamba M, Sagara J, Matsuda M, Okamura N, Makino N, Bannai S.Expression and function of cystine/glutamate transporter in neutrophils. J Leukoc Biol 2007; 81: 974-82. PMID: 17200146
- Sasaki H, Sato H, Kuriyama-Matsumura K, Sato K, Maebara K, Wang H, Tamba M, Itoh K, Yamamoto M, Bannai S. Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J Biol Chem 2002; 277: 44765-71. PMID: 12235164
- Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins.
J Biol Chem 1999; 274:11455-8. PMID: 10206947
- Sato H, Tamba M, Kuriyama-Matsumura K, Okuno S, Bannai S. Molecular cloning and expression of human xCT, the light chain of amino acid transport system xc-.
Antioxid Redox Signal. 2000; 2:665-71. PMID: 11213471
- Sato H, Tamba M, Okuno S, Sato K, Keino-Masu K, Masu M, Bannai S. Distribution of cystine/glutamate exchange transporter, system x(c)-, in the mouse brain.
J Neurosci 2002; 22: 8028-33.PMID: 12223556
- Sato H, Nomura S, Maebara K, Sato K, Tamba M, Bannai S. Transcriptional control of cystine/glutamate transporter gene by amino acid deprivation.
Biochem Biophys Res Commun 2004; 325: 109-16. PMID: 155522208
- Savaskan NE, Heckel A, Hahnen E, Engelhorn T, Doerfler A, Ganslandt O, Nimsky C, Buchfelder M, Ey¨ąpoglu IY. Small interfering RNA-mediated xCT silencing in gliomas inhibits neurodegeneration and alleviates brain edema. Nat Med 2008;14: 629-32.PMID: 18469825
- Veettil MV, Sharma-Walia N, Sadagopan S, Raghu H, Sivakumar R, Naranatt PP, Chandran B. RhoA-GTPase facilitates entry of Kaposi's sarcoma-associated herpesvirus into adherent target cells in a Src-dependent manner.
J Virol 2006; 80: 11432-46. PMID: 17005646
- Veettil MV, Sadagopan S, Sharma-Walia N, Wang FZ, Raghu H, Varga L, Chandran B. Kaposi's sarcoma-associated herpesvirus forms a multimolecular complex of integrins (alphaVbeta5, alphaVbeta3, and alpha3beta1) and CD98-xCT during infection of human dermal microvascular endothelial cells, and CD98-xCT is essential for the postentry stage of infection. J Virol 2008; 82: 12126-44. PMID: 18829766
- Wang H, Tamba M, Kimata M, Sakamoto K, Bannai S, Sato H. Expression of the activity of cystine/glutamate exchange transporter, system x(c)(-), by xCT and rBAT.
Biochem Biophys Res Commun 2003; 305: 611-8. PMID: 12763038
- Zeng Y, Li Y, Chen RS, He X, Yang L, Li W. Overexpression of xCT induces up-regulation of 14-3-3beta in Kaposi's sarcoma. Biosci Rep 2010; 30: 277-83. PMID: 20100173
EDIT HISTORY:
Created by Wei Li, 08/18/2005
Updated by Wei Li, 04/06/2006
Updated by Wei Li, 12/28/2006
Updated by Wei Li, 08/20/2008
Updated by Wei Li, 03/12/2009
Updated by Wei Li, 05/29/2011
No mutation has been reported in 32 human HPS patients who lack mutations in the seven known HPS genes (Chintala, et al).
SNPs deposited in dbSNP.
Distribution
(none)
Effect
(none)
PHENOTYPE
SLC7A11 is described in OMIM 607933.
Cystine uptake in human cancer cells is largely mediated by system Xc. xCT is relatively highly expressed in lung, colon, and central nervous system cancer cells (Huang, et al). Modulation of xCT activity is possiblely untilized in cancer therapy. Pharmacologic inhibition of system Xc remarkably inhibits the proliferation of several types of cancer cells, including lymphoma, glioma, prostate and breast cancer (Chung, et al; Doxsee, et al; Gout, et al; Narang, et al). This is thought be mediated by the depletion of GSH, which results in an increase of reactive oxygen species (ROS), and ultimately leads to caspase-dependent apoptosis (Doxsee, et al; Sakakura, et al). The apoptosis is likely mediated by c-Jun N-terminal kinase (JNK) activation. The JNK activation triggers both a caspase-dependent (caspases-9 and -3) and an ER stress-mediated (eIF2 and CHOP) pathway to induce apoptosis (Qiao, et al). In the absence of phospho-eIF2alpha, xCT expression is down-regulated that is mediated by nuclear factor ATF4, implicating the role eIF2alpha-ATF4-xCT module in neurodegeneration (Lewerenz, et al).
Disruption of xCT enhanced homotypic cell-cell adhesion and attenuated cell-extracellular matrix adhesion in an esophageal cancer cell line, KYSE150. SASP significantly inhibited both cell invasion of KYSE150 in vitro and its experimental metastasis in nude mice. Caveolin-1 was upregulated and beta-catenin was recruited to the plasma membrane when xCT was deficient, which were followed by the inhibition of beta-catenin transcriptional activity. Upregulation of caveolin-1 and inhibition of tumor cell invasion were mediated by reactive oxygen species-induced p38 MAPK activation (Chen, et al). Savaskan et al show that genetic or pharmacological inhibition of the glutamate transporter Xc system in gliomas leads to abrogated neurodegeneration, attenuated perifocal edema and prolonged survival. xCT deficiency causes unremitting inflammation because of the impaired survival of activated macrophages at the inflammatory site (Nabeyama, et al).
Kaposi's sarcoma-associated herpesvirus (KSHV, human herpesvirus 8) is the causative agent of Kaposi's sarcoma and other lymphoproliferative syndromes often associated with HIV/AIDS. xCT mediates the entry of KSHV which implicates its involvement in the infectious process of this pathogen (Kaleeba, et al; Veettil, et al (2006); Rappocciolo, et al). KSHV interacts with functionally related integrins (alphaVbeta3, alpha3beta1, and alphaVbeta5) and CD98/xCT molecules in a temporal fashion to form a multimolecular complex during the early stages of endothelial cell infection, probably mediating multiple roles in entry, signal transduction, and viral-gene expression (Veettil, et al (2008)). xCT was overexpressed in KS tissues and HHV-8-positive BCBL-1 cells. 14-3-3beta is a downstream effector of xCT in KS to mediate the cell proliferation (Zeng, et al). KSHV-encoded microRNAs (KSHV miRNAs) upregulate xCT expression by macrophages and endothelial cells, largely through miR-K12-11 suppression of BACH-1-a negative regulator of transcription recognizing antioxidant response elements within gene promoters (Qin, et al).
REFERENCE
- Chen RS, Song YM, Zhou ZY, Tong T, Li Y, Fu M, Guo XL, Dong LJ, He X, Qiao HX, Zhan QM, Li W. Disruption of xCT inhibits cancer cell metastasis via the caveolin-1/beta-catenin pathway. Oncogene 2009; 28: 599-609. PMID: 19015640
- Chintala S, Li W, Lamoreux ML, Ito S, Wakamatsu K, Sviderskaya EV, Bennett DC, Park YM, Gahl WA, Huizing M, Spritz RA, Ben S, Novak EK, Tan J, Swank RT.
Slc7a11 gene controls production of pheomelanin pigment and proliferation of cultured cells. Proc Natl Acad Sci U S A 2005; 102: 10964-9. PMID: 16037214
- Chung WJ, Lyons SA, Nelson GM, Hamza H, Gladson CL, Gillespie GY, Sontheimer H. Inhibition of cystine uptake disrupts the growth of primary brain tumors.
J Neurosci 2005; 25: 7101-10.
PMID: 16079392
- Doxsee DW, Gout PW, Kurita T, Lo M, Buckley AR, Wang Y, Xue H, Karp CM, Cutz JC, Cunha GR, Wang YZ. Sulfasalazine-induced cystine starvation: potential use for prostate cancer therapy. Prostate 2007; 67: 162-71.
PMID: 17075799
- Dun Y, Mysona B, Van Ells T, Amarnath L, Shamsul Ola M, Ganapathy V, Smith SB. Expression of the cystine-glutamate exchanger (x(c) (-)) in retinal ganglion cells and regulation by nitric oxide and oxidative stress. Cell Tissue Res 2006; 324: 189-202. PMID: 16444546
- Gasol E, Jimenez-Vidal M, Chillaron J, Zorzano A, Palacin M. Membrane topology of system xc- light subunit reveals a re-entrant loop with substrate-restricted accessibility.
J Biol Chem 2004; 279: 31228-36. PMID: 15151999
- Gout PW, Buckley AR, Simms CR, Bruchovsky N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the x(c)- cystine transporter: a new action for an old drug. Leukemia. 2001; 15: 1633-40.PMID: 11587223
- Huang Y, Dai Z, Barbacioru C, Sad¨¦e W. Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity and chemoresistance.Cancer Res 2005; 65: 7446-54.
PMID: 16103098
- Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, Masuko T, Shimizu T, Ishikawa T, Kai K, Takahashi E, Imamura Y, Baba Y, Ohmura M, Suematsu M, Baba H, Saya H. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 2011; 19: 387-400. PMID: 21397861
- Jimenez-Vidal M, Gasol E, Zorzano A, Nunes V, Palacin M, Chillaron J. Thiol modification of cysteine 327 in the eighth transmembrane domain of the light subunit xCT of the heteromeric cystine/glutamate antiporter suggests close proximity to the substrate binding site/permeation pathway.
J Biol Chem 2004; 279: 11214-21. PMID: 14722095
- Kaleeba JA, Berger EA. Kaposi's sarcoma-associated herpesvirus fusion-entry receptor: cystine transporter xCT. Science 2006; 311: 1921-4. PMID: 16574866
- Lewerenz J, Maher P. Basal levels of eIF2alpha phosphorylation determine cellular antioxidant status by regulating ATF4 and xCT expression. J Biol Chem 2009; 284: 1106-15. PMID: 19017641
- Liu XX, Li XJ, Zhang B, Liang YJ, Zhou CX, Cao DX, He M, Chen GQ, He JR, Zhao Q.
MicroRNA-26b is underexpressed in human breast cancer and induces cell apoptosis by targeting SLC7A11. FEBS Lett 2011; 585: 1363-7. PMID: 21510944
- Nabeyama A, Kurita A, Asano K, Miyake Y, Yasuda T, Miura I, Nishitai G, Arakawa S, Shimizu S, Wakana S, Yoshida H, Tanaka M. xCT deficiency accelerates chemically induced tumorigenesis. Proc Natl Acad Sci U S A 2010; 107: 6436-41.
PMID: 20308543
- Narang VS, Pauletti GM, Gout PW, Buckley DJ, Buckley AR. Suppression of cystine uptake by sulfasalazine inhibits proliferation of human mammary carcinoma cells.
Anticancer Res 2003; 23: 4571-9.
PMID: 14981898
- Rappocciolo G, Hensler HR, Jais M, Reinhart TA, Pegu A, Jenkins FJ, Rinaldo CR. Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC-SIGN. J Virol 2008; 82: 4793-806.
PMID: 18337571
- Qiang W, Cahill JM, Liu J, Kuang X, Liu N, Scofield VL, Voorhees JR, Reid AJ, Yan M, Lynn WS, Wong PK. Activation of transcription factor Nrf-2 and its downstream targets in response to moloney murine leukemia virus ts1-induced thiol depletion and oxidative stress in astrocytes. J Virol 2004; 78: 11926-38.
PMID: 15479833
- Qiao HX, Hao CJ, Li Y, He X, Chen RS, Cui J, Xu ZH, Li W. JNK activation mediates the apoptosis of xCT-deficient cells. Biochem Biophys Res Commun 2008; 370: 584-8. PMID: 18395005
- Qin Z, Freitas E, Sullivan R, Mohan S, Bacelieri R, Branch D, Romano M, Kearney P, Oates J, Plaisance K, Renne R, Kaleeba J, Parsons C. Upregulation of xCT by KSHV-encoded microRNAs facilitates KSHV dissemination and persistence in an environment of oxidative stress. PLoS Pathog 2010; 6: e1000742. PMID: 20126446
- Sakakura Y, Sato H, Shiiya A, Tamba M, Sagara J, Matsuda M, Okamura N, Makino N, Bannai S.Expression and function of cystine/glutamate transporter in neutrophils. J Leukoc Biol 2007; 81: 974-82. PMID: 17200146
- Sasaki H, Sato H, Kuriyama-Matsumura K, Sato K, Maebara K, Wang H, Tamba M, Itoh K, Yamamoto M, Bannai S. Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J Biol Chem 2002; 277: 44765-71. PMID: 12235164
- Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins.
J Biol Chem 1999; 274:11455-8. PMID: 10206947
- Sato H, Tamba M, Kuriyama-Matsumura K, Okuno S, Bannai S. Molecular cloning and expression of human xCT, the light chain of amino acid transport system xc-.
Antioxid Redox Signal. 2000; 2:665-71. PMID: 11213471
- Sato H, Tamba M, Okuno S, Sato K, Keino-Masu K, Masu M, Bannai S. Distribution of cystine/glutamate exchange transporter, system x(c)-, in the mouse brain.
J Neurosci 2002; 22: 8028-33.PMID: 12223556
- Sato H, Nomura S, Maebara K, Sato K, Tamba M, Bannai S. Transcriptional control of cystine/glutamate transporter gene by amino acid deprivation.
Biochem Biophys Res Commun 2004; 325: 109-16. PMID: 155522208
- Savaskan NE, Heckel A, Hahnen E, Engelhorn T, Doerfler A, Ganslandt O, Nimsky C, Buchfelder M, Ey¨ąpoglu IY. Small interfering RNA-mediated xCT silencing in gliomas inhibits neurodegeneration and alleviates brain edema. Nat Med 2008;14: 629-32.PMID: 18469825
- Veettil MV, Sharma-Walia N, Sadagopan S, Raghu H, Sivakumar R, Naranatt PP, Chandran B. RhoA-GTPase facilitates entry of Kaposi's sarcoma-associated herpesvirus into adherent target cells in a Src-dependent manner.
J Virol 2006; 80: 11432-46. PMID: 17005646
- Veettil MV, Sadagopan S, Sharma-Walia N, Wang FZ, Raghu H, Varga L, Chandran B. Kaposi's sarcoma-associated herpesvirus forms a multimolecular complex of integrins (alphaVbeta5, alphaVbeta3, and alpha3beta1) and CD98-xCT during infection of human dermal microvascular endothelial cells, and CD98-xCT is essential for the postentry stage of infection. J Virol 2008; 82: 12126-44. PMID: 18829766
- Wang H, Tamba M, Kimata M, Sakamoto K, Bannai S, Sato H. Expression of the activity of cystine/glutamate exchange transporter, system x(c)(-), by xCT and rBAT.
Biochem Biophys Res Commun 2003; 305: 611-8. PMID: 12763038
- Zeng Y, Li Y, Chen RS, He X, Yang L, Li W. Overexpression of xCT induces up-regulation of 14-3-3beta in Kaposi's sarcoma. Biosci Rep 2010; 30: 277-83. PMID: 20100173
EDIT HISTORY:
Created by Wei Li, 08/18/2005
Updated by Wei Li, 04/06/2006
Updated by Wei Li, 12/28/2006
Updated by Wei Li, 08/20/2008
Updated by Wei Li, 03/12/2009
Updated by Wei Li, 05/29/2011
Effect
(none)
PHENOTYPE
SLC7A11 is described in OMIM 607933.
Cystine uptake in human cancer cells is largely mediated by system Xc. xCT is relatively highly expressed in lung, colon, and central nervous system cancer cells (Huang, et al). Modulation of xCT activity is possiblely untilized in cancer therapy. Pharmacologic inhibition of system Xc remarkably inhibits the proliferation of several types of cancer cells, including lymphoma, glioma, prostate and breast cancer (Chung, et al; Doxsee, et al; Gout, et al; Narang, et al). This is thought be mediated by the depletion of GSH, which results in an increase of reactive oxygen species (ROS), and ultimately leads to caspase-dependent apoptosis (Doxsee, et al; Sakakura, et al). The apoptosis is likely mediated by c-Jun N-terminal kinase (JNK) activation. The JNK activation triggers both a caspase-dependent (caspases-9 and -3) and an ER stress-mediated (eIF2 and CHOP) pathway to induce apoptosis (Qiao, et al). In the absence of phospho-eIF2alpha, xCT expression is down-regulated that is mediated by nuclear factor ATF4, implicating the role eIF2alpha-ATF4-xCT module in neurodegeneration (Lewerenz, et al).
Disruption of xCT enhanced homotypic cell-cell adhesion and attenuated cell-extracellular matrix adhesion in an esophageal cancer cell line, KYSE150. SASP significantly inhibited both cell invasion of KYSE150 in vitro and its experimental metastasis in nude mice. Caveolin-1 was upregulated and beta-catenin was recruited to the plasma membrane when xCT was deficient, which were followed by the inhibition of beta-catenin transcriptional activity. Upregulation of caveolin-1 and inhibition of tumor cell invasion were mediated by reactive oxygen species-induced p38 MAPK activation (Chen, et al). Savaskan et al show that genetic or pharmacological inhibition of the glutamate transporter Xc system in gliomas leads to abrogated neurodegeneration, attenuated perifocal edema and prolonged survival. xCT deficiency causes unremitting inflammation because of the impaired survival of activated macrophages at the inflammatory site (Nabeyama, et al).
Kaposi's sarcoma-associated herpesvirus (KSHV, human herpesvirus 8) is the causative agent of Kaposi's sarcoma and other lymphoproliferative syndromes often associated with HIV/AIDS. xCT mediates the entry of KSHV which implicates its involvement in the infectious process of this pathogen (Kaleeba, et al; Veettil, et al (2006); Rappocciolo, et al). KSHV interacts with functionally related integrins (alphaVbeta3, alpha3beta1, and alphaVbeta5) and CD98/xCT molecules in a temporal fashion to form a multimolecular complex during the early stages of endothelial cell infection, probably mediating multiple roles in entry, signal transduction, and viral-gene expression (Veettil, et al (2008)). xCT was overexpressed in KS tissues and HHV-8-positive BCBL-1 cells. 14-3-3beta is a downstream effector of xCT in KS to mediate the cell proliferation (Zeng, et al). KSHV-encoded microRNAs (KSHV miRNAs) upregulate xCT expression by macrophages and endothelial cells, largely through miR-K12-11 suppression of BACH-1-a negative regulator of transcription recognizing antioxidant response elements within gene promoters (Qin, et al).
REFERENCE
- Chen RS, Song YM, Zhou ZY, Tong T, Li Y, Fu M, Guo XL, Dong LJ, He X, Qiao HX, Zhan QM, Li W. Disruption of xCT inhibits cancer cell metastasis via the caveolin-1/beta-catenin pathway. Oncogene 2009; 28: 599-609. PMID: 19015640
- Chintala S, Li W, Lamoreux ML, Ito S, Wakamatsu K, Sviderskaya EV, Bennett DC, Park YM, Gahl WA, Huizing M, Spritz RA, Ben S, Novak EK, Tan J, Swank RT.
Slc7a11 gene controls production of pheomelanin pigment and proliferation of cultured cells. Proc Natl Acad Sci U S A 2005; 102: 10964-9. PMID: 16037214
- Chung WJ, Lyons SA, Nelson GM, Hamza H, Gladson CL, Gillespie GY, Sontheimer H. Inhibition of cystine uptake disrupts the growth of primary brain tumors.
J Neurosci 2005; 25: 7101-10.
PMID: 16079392
- Doxsee DW, Gout PW, Kurita T, Lo M, Buckley AR, Wang Y, Xue H, Karp CM, Cutz JC, Cunha GR, Wang YZ. Sulfasalazine-induced cystine starvation: potential use for prostate cancer therapy. Prostate 2007; 67: 162-71.
PMID: 17075799
- Dun Y, Mysona B, Van Ells T, Amarnath L, Shamsul Ola M, Ganapathy V, Smith SB. Expression of the cystine-glutamate exchanger (x(c) (-)) in retinal ganglion cells and regulation by nitric oxide and oxidative stress. Cell Tissue Res 2006; 324: 189-202. PMID: 16444546
- Gasol E, Jimenez-Vidal M, Chillaron J, Zorzano A, Palacin M. Membrane topology of system xc- light subunit reveals a re-entrant loop with substrate-restricted accessibility.
J Biol Chem 2004; 279: 31228-36. PMID: 15151999
- Gout PW, Buckley AR, Simms CR, Bruchovsky N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the x(c)- cystine transporter: a new action for an old drug. Leukemia. 2001; 15: 1633-40.PMID: 11587223
- Huang Y, Dai Z, Barbacioru C, Sad¨¦e W. Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity and chemoresistance.Cancer Res 2005; 65: 7446-54.
PMID: 16103098
- Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, Masuko T, Shimizu T, Ishikawa T, Kai K, Takahashi E, Imamura Y, Baba Y, Ohmura M, Suematsu M, Baba H, Saya H. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 2011; 19: 387-400. PMID: 21397861
- Jimenez-Vidal M, Gasol E, Zorzano A, Nunes V, Palacin M, Chillaron J. Thiol modification of cysteine 327 in the eighth transmembrane domain of the light subunit xCT of the heteromeric cystine/glutamate antiporter suggests close proximity to the substrate binding site/permeation pathway.
J Biol Chem 2004; 279: 11214-21. PMID: 14722095
- Kaleeba JA, Berger EA. Kaposi's sarcoma-associated herpesvirus fusion-entry receptor: cystine transporter xCT. Science 2006; 311: 1921-4. PMID: 16574866
- Lewerenz J, Maher P. Basal levels of eIF2alpha phosphorylation determine cellular antioxidant status by regulating ATF4 and xCT expression. J Biol Chem 2009; 284: 1106-15. PMID: 19017641
- Liu XX, Li XJ, Zhang B, Liang YJ, Zhou CX, Cao DX, He M, Chen GQ, He JR, Zhao Q.
MicroRNA-26b is underexpressed in human breast cancer and induces cell apoptosis by targeting SLC7A11. FEBS Lett 2011; 585: 1363-7. PMID: 21510944
- Nabeyama A, Kurita A, Asano K, Miyake Y, Yasuda T, Miura I, Nishitai G, Arakawa S, Shimizu S, Wakana S, Yoshida H, Tanaka M. xCT deficiency accelerates chemically induced tumorigenesis. Proc Natl Acad Sci U S A 2010; 107: 6436-41.
PMID: 20308543
- Narang VS, Pauletti GM, Gout PW, Buckley DJ, Buckley AR. Suppression of cystine uptake by sulfasalazine inhibits proliferation of human mammary carcinoma cells.
Anticancer Res 2003; 23: 4571-9.
PMID: 14981898
- Rappocciolo G, Hensler HR, Jais M, Reinhart TA, Pegu A, Jenkins FJ, Rinaldo CR. Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC-SIGN. J Virol 2008; 82: 4793-806.
PMID: 18337571
- Qiang W, Cahill JM, Liu J, Kuang X, Liu N, Scofield VL, Voorhees JR, Reid AJ, Yan M, Lynn WS, Wong PK. Activation of transcription factor Nrf-2 and its downstream targets in response to moloney murine leukemia virus ts1-induced thiol depletion and oxidative stress in astrocytes. J Virol 2004; 78: 11926-38.
PMID: 15479833
- Qiao HX, Hao CJ, Li Y, He X, Chen RS, Cui J, Xu ZH, Li W. JNK activation mediates the apoptosis of xCT-deficient cells. Biochem Biophys Res Commun 2008; 370: 584-8. PMID: 18395005
- Qin Z, Freitas E, Sullivan R, Mohan S, Bacelieri R, Branch D, Romano M, Kearney P, Oates J, Plaisance K, Renne R, Kaleeba J, Parsons C. Upregulation of xCT by KSHV-encoded microRNAs facilitates KSHV dissemination and persistence in an environment of oxidative stress. PLoS Pathog 2010; 6: e1000742. PMID: 20126446
- Sakakura Y, Sato H, Shiiya A, Tamba M, Sagara J, Matsuda M, Okamura N, Makino N, Bannai S.Expression and function of cystine/glutamate transporter in neutrophils. J Leukoc Biol 2007; 81: 974-82. PMID: 17200146
- Sasaki H, Sato H, Kuriyama-Matsumura K, Sato K, Maebara K, Wang H, Tamba M, Itoh K, Yamamoto M, Bannai S. Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J Biol Chem 2002; 277: 44765-71. PMID: 12235164
- Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins.
J Biol Chem 1999; 274:11455-8. PMID: 10206947
- Sato H, Tamba M, Kuriyama-Matsumura K, Okuno S, Bannai S. Molecular cloning and expression of human xCT, the light chain of amino acid transport system xc-.
Antioxid Redox Signal. 2000; 2:665-71. PMID: 11213471
- Sato H, Tamba M, Okuno S, Sato K, Keino-Masu K, Masu M, Bannai S. Distribution of cystine/glutamate exchange transporter, system x(c)-, in the mouse brain.
J Neurosci 2002; 22: 8028-33.PMID: 12223556
- Sato H, Nomura S, Maebara K, Sato K, Tamba M, Bannai S. Transcriptional control of cystine/glutamate transporter gene by amino acid deprivation.
Biochem Biophys Res Commun 2004; 325: 109-16. PMID: 155522208
- Savaskan NE, Heckel A, Hahnen E, Engelhorn T, Doerfler A, Ganslandt O, Nimsky C, Buchfelder M, Ey¨ąpoglu IY. Small interfering RNA-mediated xCT silencing in gliomas inhibits neurodegeneration and alleviates brain edema. Nat Med 2008;14: 629-32.PMID: 18469825
- Veettil MV, Sharma-Walia N, Sadagopan S, Raghu H, Sivakumar R, Naranatt PP, Chandran B. RhoA-GTPase facilitates entry of Kaposi's sarcoma-associated herpesvirus into adherent target cells in a Src-dependent manner.
J Virol 2006; 80: 11432-46. PMID: 17005646
- Veettil MV, Sadagopan S, Sharma-Walia N, Wang FZ, Raghu H, Varga L, Chandran B. Kaposi's sarcoma-associated herpesvirus forms a multimolecular complex of integrins (alphaVbeta5, alphaVbeta3, and alpha3beta1) and CD98-xCT during infection of human dermal microvascular endothelial cells, and CD98-xCT is essential for the postentry stage of infection. J Virol 2008; 82: 12126-44. PMID: 18829766
- Wang H, Tamba M, Kimata M, Sakamoto K, Bannai S, Sato H. Expression of the activity of cystine/glutamate exchange transporter, system x(c)(-), by xCT and rBAT.
Biochem Biophys Res Commun 2003; 305: 611-8. PMID: 12763038
- Zeng Y, Li Y, Chen RS, He X, Yang L, Li W. Overexpression of xCT induces up-regulation of 14-3-3beta in Kaposi's sarcoma. Biosci Rep 2010; 30: 277-83. PMID: 20100173
EDIT HISTORY:
Created by Wei Li, 08/18/2005
Updated by Wei Li, 04/06/2006
Updated by Wei Li, 12/28/2006
Updated by Wei Li, 08/20/2008
Updated by Wei Li, 03/12/2009
Updated by Wei Li, 05/29/2011
PHENOTYPE
SLC7A11 is described in OMIM 607933.
Cystine uptake in human cancer cells is largely mediated by system Xc. xCT is relatively highly expressed in lung, colon, and central nervous system cancer cells (Huang, et al). Modulation of xCT activity is possiblely untilized in cancer therapy. Pharmacologic inhibition of system Xc remarkably inhibits the proliferation of several types of cancer cells, including lymphoma, glioma, prostate and breast cancer (Chung, et al; Doxsee, et al; Gout, et al; Narang, et al). This is thought be mediated by the depletion of GSH, which results in an increase of reactive oxygen species (ROS), and ultimately leads to caspase-dependent apoptosis (Doxsee, et al; Sakakura, et al). The apoptosis is likely mediated by c-Jun N-terminal kinase (JNK) activation. The JNK activation triggers both a caspase-dependent (caspases-9 and -3) and an ER stress-mediated (eIF2 and CHOP) pathway to induce apoptosis (Qiao, et al). In the absence of phospho-eIF2alpha, xCT expression is down-regulated that is mediated by nuclear factor ATF4, implicating the role eIF2alpha-ATF4-xCT module in neurodegeneration (Lewerenz, et al).
Disruption of xCT enhanced homotypic cell-cell adhesion and attenuated cell-extracellular matrix adhesion in an esophageal cancer cell line, KYSE150. SASP significantly inhibited both cell invasion of KYSE150 in vitro and its experimental metastasis in nude mice. Caveolin-1 was upregulated and beta-catenin was recruited to the plasma membrane when xCT was deficient, which were followed by the inhibition of beta-catenin transcriptional activity. Upregulation of caveolin-1 and inhibition of tumor cell invasion were mediated by reactive oxygen species-induced p38 MAPK activation (Chen, et al). Savaskan et al show that genetic or pharmacological inhibition of the glutamate transporter Xc system in gliomas leads to abrogated neurodegeneration, attenuated perifocal edema and prolonged survival. xCT deficiency causes unremitting inflammation because of the impaired survival of activated macrophages at the inflammatory site (Nabeyama, et al).
Kaposi's sarcoma-associated herpesvirus (KSHV, human herpesvirus 8) is the causative agent of Kaposi's sarcoma and other lymphoproliferative syndromes often associated with HIV/AIDS. xCT mediates the entry of KSHV which implicates its involvement in the infectious process of this pathogen (Kaleeba, et al; Veettil, et al (2006); Rappocciolo, et al). KSHV interacts with functionally related integrins (alphaVbeta3, alpha3beta1, and alphaVbeta5) and CD98/xCT molecules in a temporal fashion to form a multimolecular complex during the early stages of endothelial cell infection, probably mediating multiple roles in entry, signal transduction, and viral-gene expression (Veettil, et al (2008)). xCT was overexpressed in KS tissues and HHV-8-positive BCBL-1 cells. 14-3-3beta is a downstream effector of xCT in KS to mediate the cell proliferation (Zeng, et al). KSHV-encoded microRNAs (KSHV miRNAs) upregulate xCT expression by macrophages and endothelial cells, largely through miR-K12-11 suppression of BACH-1-a negative regulator of transcription recognizing antioxidant response elements within gene promoters (Qin, et al).
REFERENCE
- Chen RS, Song YM, Zhou ZY, Tong T, Li Y, Fu M, Guo XL, Dong LJ, He X, Qiao HX, Zhan QM, Li W. Disruption of xCT inhibits cancer cell metastasis via the caveolin-1/beta-catenin pathway. Oncogene 2009; 28: 599-609. PMID: 19015640
- Chintala S, Li W, Lamoreux ML, Ito S, Wakamatsu K, Sviderskaya EV, Bennett DC, Park YM, Gahl WA, Huizing M, Spritz RA, Ben S, Novak EK, Tan J, Swank RT. Slc7a11 gene controls production of pheomelanin pigment and proliferation of cultured cells. Proc Natl Acad Sci U S A 2005; 102: 10964-9. PMID: 16037214
- Chung WJ, Lyons SA, Nelson GM, Hamza H, Gladson CL, Gillespie GY, Sontheimer H. Inhibition of cystine uptake disrupts the growth of primary brain tumors. J Neurosci 2005; 25: 7101-10. PMID: 16079392
- Doxsee DW, Gout PW, Kurita T, Lo M, Buckley AR, Wang Y, Xue H, Karp CM, Cutz JC, Cunha GR, Wang YZ. Sulfasalazine-induced cystine starvation: potential use for prostate cancer therapy. Prostate 2007; 67: 162-71. PMID: 17075799
- Dun Y, Mysona B, Van Ells T, Amarnath L, Shamsul Ola M, Ganapathy V, Smith SB. Expression of the cystine-glutamate exchanger (x(c) (-)) in retinal ganglion cells and regulation by nitric oxide and oxidative stress. Cell Tissue Res 2006; 324: 189-202. PMID: 16444546
- Gasol E, Jimenez-Vidal M, Chillaron J, Zorzano A, Palacin M. Membrane topology of system xc- light subunit reveals a re-entrant loop with substrate-restricted accessibility. J Biol Chem 2004; 279: 31228-36. PMID: 15151999
- Gout PW, Buckley AR, Simms CR, Bruchovsky N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the x(c)- cystine transporter: a new action for an old drug. Leukemia. 2001; 15: 1633-40.PMID: 11587223
- Huang Y, Dai Z, Barbacioru C, Sad¨¦e W. Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity and chemoresistance.Cancer Res 2005; 65: 7446-54. PMID: 16103098
- Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, Masuko T, Shimizu T, Ishikawa T, Kai K, Takahashi E, Imamura Y, Baba Y, Ohmura M, Suematsu M, Baba H, Saya H. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 2011; 19: 387-400. PMID: 21397861
- Jimenez-Vidal M, Gasol E, Zorzano A, Nunes V, Palacin M, Chillaron J. Thiol modification of cysteine 327 in the eighth transmembrane domain of the light subunit xCT of the heteromeric cystine/glutamate antiporter suggests close proximity to the substrate binding site/permeation pathway. J Biol Chem 2004; 279: 11214-21. PMID: 14722095
- Kaleeba JA, Berger EA. Kaposi's sarcoma-associated herpesvirus fusion-entry receptor: cystine transporter xCT. Science 2006; 311: 1921-4. PMID: 16574866
- Lewerenz J, Maher P. Basal levels of eIF2alpha phosphorylation determine cellular antioxidant status by regulating ATF4 and xCT expression. J Biol Chem 2009; 284: 1106-15. PMID: 19017641
- Liu XX, Li XJ, Zhang B, Liang YJ, Zhou CX, Cao DX, He M, Chen GQ, He JR, Zhao Q. MicroRNA-26b is underexpressed in human breast cancer and induces cell apoptosis by targeting SLC7A11. FEBS Lett 2011; 585: 1363-7. PMID: 21510944
- Nabeyama A, Kurita A, Asano K, Miyake Y, Yasuda T, Miura I, Nishitai G, Arakawa S, Shimizu S, Wakana S, Yoshida H, Tanaka M. xCT deficiency accelerates chemically induced tumorigenesis. Proc Natl Acad Sci U S A 2010; 107: 6436-41. PMID: 20308543
- Narang VS, Pauletti GM, Gout PW, Buckley DJ, Buckley AR. Suppression of cystine uptake by sulfasalazine inhibits proliferation of human mammary carcinoma cells. Anticancer Res 2003; 23: 4571-9. PMID: 14981898
- Rappocciolo G, Hensler HR, Jais M, Reinhart TA, Pegu A, Jenkins FJ, Rinaldo CR. Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC-SIGN. J Virol 2008; 82: 4793-806. PMID: 18337571
- Qiang W, Cahill JM, Liu J, Kuang X, Liu N, Scofield VL, Voorhees JR, Reid AJ, Yan M, Lynn WS, Wong PK. Activation of transcription factor Nrf-2 and its downstream targets in response to moloney murine leukemia virus ts1-induced thiol depletion and oxidative stress in astrocytes. J Virol 2004; 78: 11926-38. PMID: 15479833
- Qiao HX, Hao CJ, Li Y, He X, Chen RS, Cui J, Xu ZH, Li W. JNK activation mediates the apoptosis of xCT-deficient cells. Biochem Biophys Res Commun 2008; 370: 584-8. PMID: 18395005
- Qin Z, Freitas E, Sullivan R, Mohan S, Bacelieri R, Branch D, Romano M, Kearney P, Oates J, Plaisance K, Renne R, Kaleeba J, Parsons C. Upregulation of xCT by KSHV-encoded microRNAs facilitates KSHV dissemination and persistence in an environment of oxidative stress. PLoS Pathog 2010; 6: e1000742. PMID: 20126446
- Sakakura Y, Sato H, Shiiya A, Tamba M, Sagara J, Matsuda M, Okamura N, Makino N, Bannai S.Expression and function of cystine/glutamate transporter in neutrophils. J Leukoc Biol 2007; 81: 974-82. PMID: 17200146
- Sasaki H, Sato H, Kuriyama-Matsumura K, Sato K, Maebara K, Wang H, Tamba M, Itoh K, Yamamoto M, Bannai S. Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J Biol Chem 2002; 277: 44765-71. PMID: 12235164
- Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem 1999; 274:11455-8. PMID: 10206947
- Sato H, Tamba M, Kuriyama-Matsumura K, Okuno S, Bannai S. Molecular cloning and expression of human xCT, the light chain of amino acid transport system xc-. Antioxid Redox Signal. 2000; 2:665-71. PMID: 11213471
- Sato H, Tamba M, Okuno S, Sato K, Keino-Masu K, Masu M, Bannai S. Distribution of cystine/glutamate exchange transporter, system x(c)-, in the mouse brain. J Neurosci 2002; 22: 8028-33.PMID: 12223556
- Sato H, Nomura S, Maebara K, Sato K, Tamba M, Bannai S. Transcriptional control of cystine/glutamate transporter gene by amino acid deprivation. Biochem Biophys Res Commun 2004; 325: 109-16. PMID: 155522208
- Savaskan NE, Heckel A, Hahnen E, Engelhorn T, Doerfler A, Ganslandt O, Nimsky C, Buchfelder M, Ey¨ąpoglu IY. Small interfering RNA-mediated xCT silencing in gliomas inhibits neurodegeneration and alleviates brain edema. Nat Med 2008;14: 629-32.PMID: 18469825
- Veettil MV, Sharma-Walia N, Sadagopan S, Raghu H, Sivakumar R, Naranatt PP, Chandran B. RhoA-GTPase facilitates entry of Kaposi's sarcoma-associated herpesvirus into adherent target cells in a Src-dependent manner. J Virol 2006; 80: 11432-46. PMID: 17005646
- Veettil MV, Sadagopan S, Sharma-Walia N, Wang FZ, Raghu H, Varga L, Chandran B. Kaposi's sarcoma-associated herpesvirus forms a multimolecular complex of integrins (alphaVbeta5, alphaVbeta3, and alpha3beta1) and CD98-xCT during infection of human dermal microvascular endothelial cells, and CD98-xCT is essential for the postentry stage of infection. J Virol 2008; 82: 12126-44. PMID: 18829766
- Wang H, Tamba M, Kimata M, Sakamoto K, Bannai S, Sato H. Expression of the activity of cystine/glutamate exchange transporter, system x(c)(-), by xCT and rBAT. Biochem Biophys Res Commun 2003; 305: 611-8. PMID: 12763038
- Zeng Y, Li Y, Chen RS, He X, Yang L, Li W. Overexpression of xCT induces up-regulation of 14-3-3beta in Kaposi's sarcoma. Biosci Rep 2010; 30: 277-83. PMID: 20100173
EDIT HISTORY:
Created by Wei Li, 08/18/2005
Updated by Wei Li, 04/06/2006
Updated by Wei Li, 12/28/2006
Updated by Wei Li, 08/20/2008
Updated by Wei Li, 03/12/2009
Updated by Wei Li, 05/29/2011